Risk Factors for a Higher Symptom Burden in Patients With Cancer During the COVID-19 Pandemic
Objectives: To evaluate for subgroups of patients with distinct symptom profiles and differences in demographic and clinical characteristics and stress and resilience among these subgroups.
Sample & Setting: 1,145 patients with cancer aged 18 years or older completed a survey online. Data were collected between May 2020 and February 2021.
Methods & Variables: Patients completed measures for depression, state anxiety, cognitive function, morning fatigue, evening fatigue, morning energy, evening energy, sleep disturbance, pain, stress, and resilience. Latent class profile analysis was used to identify subgroups of patients with distinct symptom profiles. Differences among the subgroups on study measures were evaluated using parametric and nonparametric tests.
Results: Four distinct profiles were identified (none, low, high, and very high). Patients in the high and very high classes reported clinically meaningful levels of all nine symptoms. Differences among the four profiles for stress and resilience exhibited a dose–response effect.
Implications for Nursing: Findings can serve as benchmark data of the symptom burden of patients with cancer following the COVID-19 pandemic.
Jump to a section
As a result of the COVID-19 pandemic that began in March 2020, cancer care underwent a dramatic transformation (Ashbury, 2021). With the implementation of isolation and mitigation procedures and limited access to inpatient and outpatient services, many patients with cancer received care using telehealth approaches (Ashbury, 2021; Singh et al., 2021). On an individual level, patients experienced significant stress associated with fears of contracting the COVID-19 virus, disruptions in cancer treatments and follow-up appointments, and financial concerns associated with job losses and decreases in income, as well as social isolation and loneliness (Ashbury, 2021).
Background
Cancer Care During the COVID-19 Pandemic
The COVID-19 pandemic created changes in individuals’ healthcare behaviors to accommodate fears associated with contracting the COVID-19 virus and social distancing procedures (Moraliyage et al., 2021). For example, in a population-based study in the United Kingdom (Quinn-Scoggins et al., 2021), of the 40.1% of participants who experienced a symptom suggestive of cancer, 44.8% did not contact their primary care provider. The major reasons for not seeking care included fear of going to the hospital, worries about wasting clinicians’ time, and concerns about putting strain on healthcare services. For patients with cancer and survivors, inconsistent guidelines from public health officials and professional organizations on cancer care created an atmosphere of uncertainty regarding treatment decisions (Mauri et al., 2020; Saini et al., 2020). In addition, delays in cancer screening, suspension of clinical trials, and postponements of ongoing or planned therapy added to patients’ stress (Mauri et al., 2020; Moraliyage et al., 2021; Saini et al., 2020; Venkatesulu et al., 2021). Patients with cancer also experienced significant reductions in access to social services and supportive care, which worsened feelings of stress and loneliness (Aapro et al., 2021; Gallagher et al., 2021).
Symptoms and Stress During the COVID-19 Pandemic
Prior to the COVID-19 pandemic, patients with cancer reported an average of nine unrelieved symptoms (Mazor et al., 2019). The most common symptoms included fatigue, sleep disturbance, depression, anxiety, and pain. Of note, a higher symptom burden was associated with higher levels of perceived stress. In a previous study that evaluated symptom burden during the COVID-19 pandemic (Miaskowski et al., 2020), patients with cancer in the stressed group reported clinically meaningful levels of depression, anxiety, fatigue, and sleep disturbance, as well as significant decrements in energy and cognitive function. However, a large amount of interindividual variability existed in the scores for all symptoms. In a qualitative study of patients who had access to online cancer forums (Colomer-Lahiguera et al., 2021), an analysis of 230 posts identified the most common emotions associated with the COVID-19 pandemic to be as follows: feeling fear/panic, feeling lost, being stressed/anxious, being sad/depressed, feeling ignored/discarded, being upset, or feeling alone. However, in a longitudinal study of older patients with breast cancer and noncancer controls (Rentscher et al., 2021), no between-group differences were found in depression, anxiety, and loneliness scores. Of note, in both groups, as loneliness increased, depression and anxiety levels increased.
As reported in previous studies of patients with cancer (Allemann-Su et al., 2023; Calvo-Schimmel et al., 2022; Hammer et al., 2022; Huang et al., 2022; Lin et al., 2022; Shin et al., 2022), a large amount of interindividual variability exists in patients’ symptom experiences. Person-centered analytic approaches, such as latent variable modeling (Muthén, 2002), allow for the identification of subgroups of patients with distinct symptom profiles. Once these subgroups are identified, risk factors associated with a worse symptom profile can be determined.
During the COVID-19 pandemic, patients with cancer experienced clinically meaningful levels of fatigue, sleep disturbance, depression, anxiety, cognitive impairment, and pain (Miaskowski et al., 2020). However, no studies were identified that evaluated for interindividual variability in patients’ symptom experiences for these common co-occurring symptoms and associated risk factors during the COVID-19 pandemic. Therefore, in a sample of patients with cancer (N = 1,145) who were assessed from May 2020 to February 2021, the purposes of this study were to use latent class profile analysis (LCPA) to evaluate for subgroups of patients with distinct symptom profiles and to evaluate for differences in demographic and clinical characteristics and stress scores among these subgroups. The researchers hypothesized that patients with a worse symptom profile will report higher levels of global- and cancer-specific stress, as well as higher levels of social isolation and loneliness and lower levels of resilience.
Methods
Theoretical Framework
The theory of symptom management served as the theoretical framework for this study (Weiss et al., 2023). Interindividual variability in patients’ experiences with the most common co-occurring symptoms associated with cancer and its treatments were evaluated in the context of person, clinical, and stress characteristics.
Sample and Setting
Patients were recruited from a registry of individuals who participated in previous symptom management studies (CA187160, CA212064, CA151692); electronic health record searches for patients with cancer diagnoses at the University of California, San Francisco (UCSF); Mount Sinai Medical Center and Columbia University Irving Medical Center, both in New York, New York; and the Dr. Susan Love Foundation for Breast Cancer Research. Potential participants received an email that briefly detailed the study and provided a link that directed them to the study’s enrollment page, which explained the purpose of the study, the time frame for survey completion, and information about participating in the research. This study was exempt from requiring written informed consent by the UCSF Institutional Review Board and from each of the participating institutions.
Patients were included if they were aged 18 years or older; were able to read, write, and understand English; had a diagnosis of cancer; were able to complete the study questionnaires online; and had consented to participate by completing the survey. Of the 1,908 patients who began the survey, 1,145 patients completed the survey data presented in this article (60% completion rate).
Recruitment, Survey Administration, and Study Measures
Beginning May 27, 2020, emails were sent to potential patients. Responses until February 22, 2021, are presented in this article. Patients were asked to complete the survey within two weeks. One reminder was sent to the patients who did not respond to the initial request to complete the survey after two weeks.
Patients were asked to answer all the survey questions in relationship to their experiences during the past 14 days. The survey took about 60 minutes to complete. Patients were advised that completing the survey in one sitting was preferred, but they could take as many breaks as needed. All the study instruments were completed online using REDCap.
Instruments
Demographic and clinical characteristics: Patients completed a demographic questionnaire, Karnofsky Performance Status Scale (Karnofsky, 1977), Self-Administered Comorbidity Questionnaire (Sangha et al., 2003), and International Physical Activity Questionnaire (Craig et al., 2003; Hallal & Victora, 2004). In addition, patients answered questions about their height and weight, cancer diagnosis, previous and current cancer treatments, presence of metastatic disease, and occurrence of the COVID-19 virus.
Symptom measures: The 20-item Center for Epidemiological Studies–Depression scale evaluates major symptoms associated with the clinical syndrome of depression. Total scores range from 0 to 60, with scores of 16 or greater indicating the need for individuals to seek clinical evaluation for major depression (Radloff, 1977). The Cronbach’s alpha was 0.92.
The 20 items on the Spielberger State-Trait Anxiety Inventories (STAI-T and STAI-S) were summed for each scale to create a score ranging from 20 to 80. The STAI-S measures an individual’s temporary anxiety response or how anxious or tense an individual is in a specific situation. The STAI-T measures an individual’s predisposition to anxiety as part of their personality. Cutoff scores of 31.8 or greater and 32.2 or greater indicate a high level of trait and state anxiety, respectively (Spielberger et al., 1983). Cronbach’s alphas for the STAI-T and STAI-S were 0.94 and 0.97, respectively. State anxiety scores were used in the LCPA.
The 13-item Attentional Function Index measures an individual’s perceived effectiveness in performing daily activities that are supported by attention, working memory, and executive functions (Cimprich et al., 2011). A higher total mean score on a 0–10 numeric rating scale (NRS) indicates a greater capacity to direct attention (Cimprich et al., 2011). Total scores are grouped into categories of attentional function, with a score of less than 5 indicating low function, a score of 5–7.5 indicating moderate function, and a score of greater than 7.5 indicating high function (Cimprich et al., 2005). The Cronbach’s alpha was 0.93.
The 18-item Lee Fatigue Scale assesses physical fatigue and energy on a 0–10 NRS (Lee et al., 1991). Mean scores on the 13 fatigue items and the 5 energy items are used to calculate total fatigue and energy scores. Higher scores indicate greater fatigue severity and higher levels of energy. Using separate Lee Fatigue Scale questionnaires, patients were asked to rate each item based on how they felt within 30 minutes of awakening (morning fatigue and morning energy) and going to bed (evening fatigue and evening energy). The established cutoff scores on the Lee Fatigue Scale for clinically meaningful levels of fatigue are 3.2 or greater for morning fatigue, 5.6 or greater for evening fatigue, 6.2 or lower for morning energy, and 3.5 or lower for evening energy (Fletcher et al., 2008). The Cronbach’s alphas were 0.97 for morning fatigue, 0.94 for evening fatigue, 0.96 for morning energy, and 0.93 for evening energy.
The 21-item General Sleep Disturbance Scale (GSDS) assesses sleep quality using an NRS from 0 (never) to 7 (every day). Scores from the seven subscale are summed for a total GSDS score, which ranges from 0 (no disturbance) to 147 (extreme sleep disturbance). A score of 43 or greater indicates a clinically meaningful level of sleep disturbance (Fletcher et al., 2008). Its Cronbach’s alpha was 0.86.
Pain occurrence was evaluated using the Brief Pain Inventory (Daut et al., 1983). Patients who responded “yes” to the question about having pain were asked to rate the intensity of their worst pain using an NRS from 0 (none) to 10 (excruciating).
Stress and resilience measures: The 22-item Impact of Event Scale–Revised (IES-R) was used to measure distress associated with cancer and its treatment and the COVID-19 pandemic (Weiss & Marmar, 1997). Patients rated each item based on how distressing they found a potential difficulty during the past 14 days “with respect to their cancer and its treatment and the COVID-19 pandemic.” Responses are summed to calculate a total IES-R score. For the IES-R total score, sum scores of 24 or greater indicate clinically meaningful post-traumatic symptomatology, and scores of 33 or greater indicate probable post-traumatic stress disorder (Creamer et al., 2003; Morina et al., 2013). Its Cronbach’s alpha was 0.93.
The 10-item Perceived Stress Scale (PSS) was used to measure global perceived stress according to the degree that life circumstances were appraised as stressful during the past 14 days (Cohen et al., 1983). Total PSS scores range from 0 to 40. Its Cronbach’s alpha was 0.91.
The six-item Social Isolation Scale evaluates an individual’s perceptions of connectedness and belongingness (Nicholson et al., 2020). Scores of 10–15 suggest that an individual is at risk for social isolation, and a score of 9 or lower indicates social isolation. Its Cronbach’s alpha was 0.71.
The 20-item University of California, Los Angeles (UCLA), Loneliness Scale measures an individual’s subjective feelings of loneliness and social isolation (Russell, 1996; Russell et al., 1978, 1980). A score of 36 represents a normative value for the general population (Knight et al., 1988). Its Cronbach’s alpha was 0.95.
The 10-item Connor-Davidson Resilience Scale evaluates an individual’s personal ability to handle adversity (e.g., “I am able to adapt when changes occur”) (Campbell-Sills & Stein, 2007). Total scores range from 0 to 40, with higher scores indicating higher self-perceived resilience. The normative adult mean score in the United States is 31.8 (SD = 5.4) (Campbell-Sills et al., 2009). Its Cronbach’s alpha was 0.91.
Data Analysis
Survey responses were stored on a secure UCSF server. Data were downloaded from REDCap into IBM SPSS Statistics, version 28.0, for subsequent analyses.
LCPA was used to identify subgroups of patients (i.e., latent classes) with similar experiences for nine symptoms (depression, state anxiety, cognitive function, morning fatigue, evening fatigue, morning energy, evening energy, sleep disturbance, and worst pain) (Vermunt & Magidson, 2002). Latent class models often use categorical variables (Lanza et al., 2003). When continuous variables are analyzed as in the current study, LCPA is used. However, one of the continuous variables in this study, worst pain, had many scores of 0 because a number of patients did not report pain. This large number of 0 scores was accommodated by modeling worst pain as a two-part variable. In this type of model, one part represents the difference between those who did and did not report the variable, and the second part differentiates among those who reported any pain on the remaining portion of the NRS (i.e., the 1–10 part of the NRS) (Muthén & Muthén, 1998–2010).
LCPA was performed using Mplus, version 8.5 (Muthén & Muthén, 1998–2010). The Bayesian information criterion (BIC), Vuong–Lo–Mendell–Rubin likelihood ratio test (VLMR), and entropy were evaluated to identify the final number of latent classes. In this analysis, the model that fits the data best has the lowest BIC and/or VLMR (Nylund et al., 2007). Better-fitting models produce higher entropy values (Celeux & Soromenho, 1996). Finally, well-fitting models make sense conceptually, and the estimated classes differ on variables not used in the generation of the model (Nylund et al., 2007). Estimation was performed with robust maximum likelihood, and missingness was accommodated by using the expectation maximization algorithm (Muthén & Shedden, 1999). Because of the inclusion of a categorical variable (the binary variable for the occurrence of pain versus no pain), rectangular numeric integration with 15 integration points was employed for logit estimation.
Parametric and nonparametric tests were used to evaluate differences among the latent classes in demographic, clinical, stress, and resilience characteristics. A p value of < 0.05 was considered statistically significant. Post hoc contrasts were done using a Bonferroni corrected p value of 0.008 (0.05/6 possible pairwise contrasts).
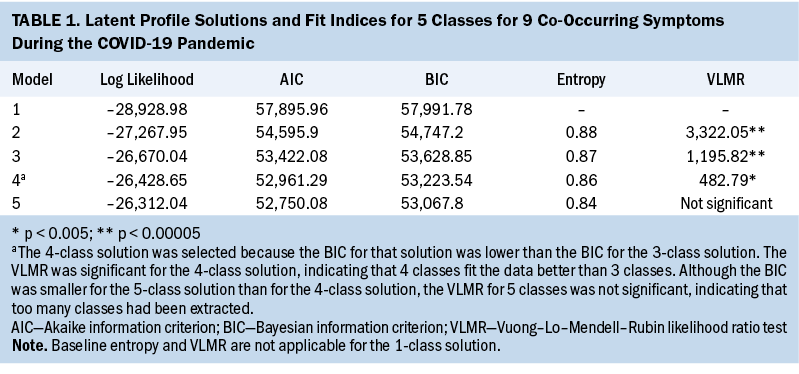
Results
A four-class solution was selected because the BIC for that solution was lower than the BIC for the three-class solution (see Table 1). In addition, the four-class solution VLMR was significant, indicating that four classes fit the data better than three classes. Although the BIC was smaller for the five-class solution than for the four-class solution, the VLMR for the five-class solution was not significant, indicating that too many classes were extracted. Using clinically meaningful cutoff scores for the symptom measures to name the classes (see Table 2), of the 1,145 survivors in this study, 327 (29%) were in the none class, 432 (38%) were in the low class, 296 (26%) were in the high class, and 90 (8%) were in the very high class.
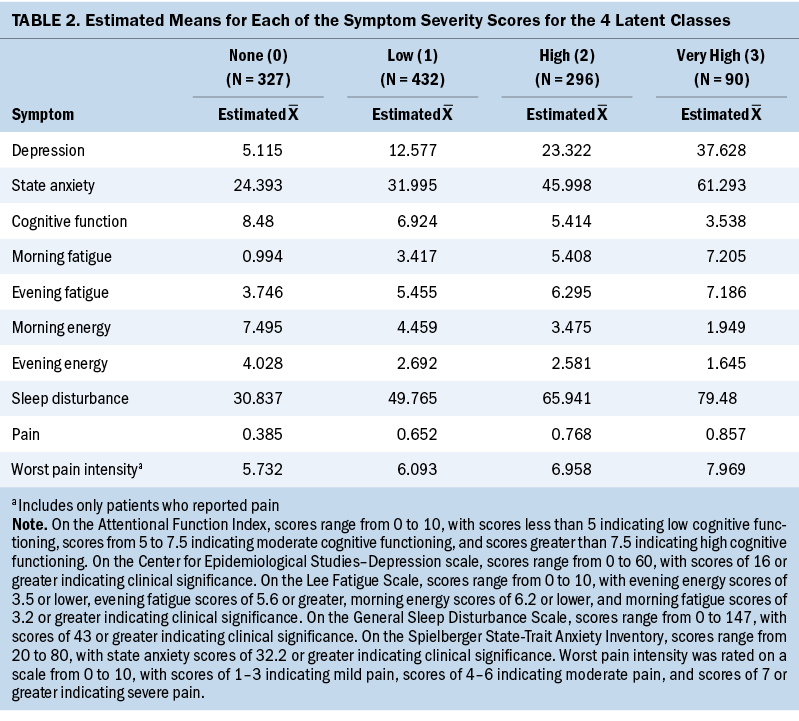
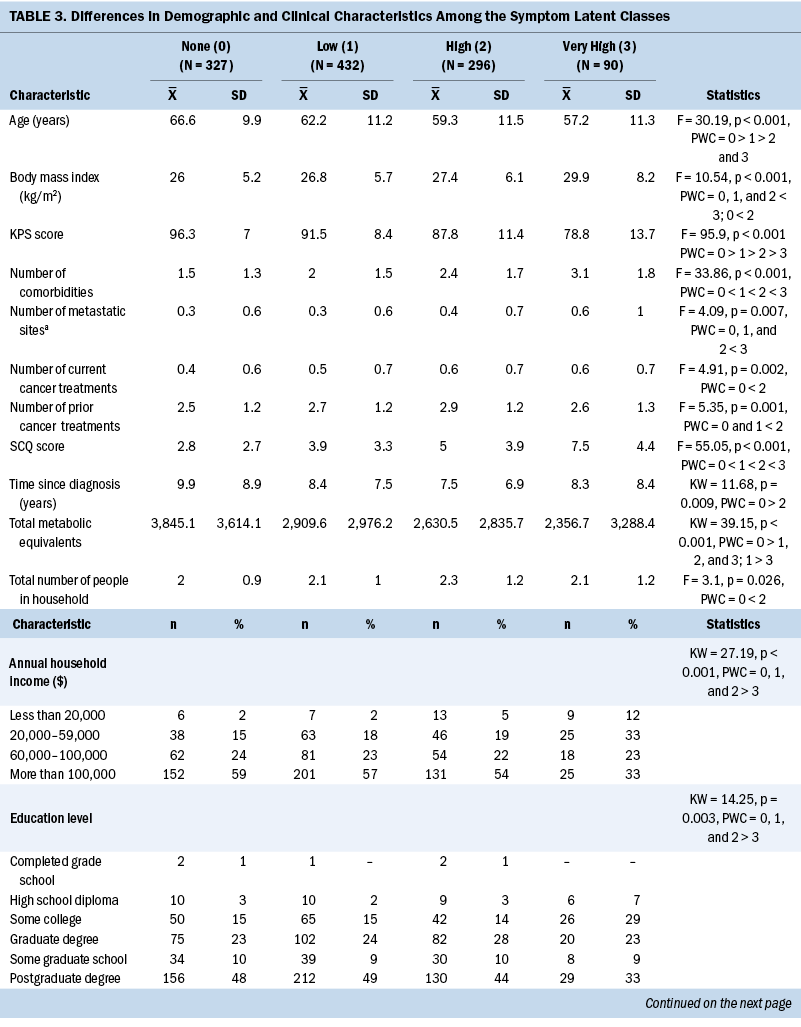
Demographic and Clinical Characteristics
As shown in Table 3, compared to the none class, the other three classes were more likely to be female, report a lower level of exercise, and report back pain. Compared to the other classes, the very high class was younger, had a lower level of education, had a lower annual household income, had a higher body mass index (BMI), and had a higher number of metastatic disease sites. Differences among the four classes in Karnofsky Performance Status Scale scores, number of comorbidities, Self-Administered Comorbidity Questionnaire scores, and occurrence rates for self-reported depression followed similar patterns.
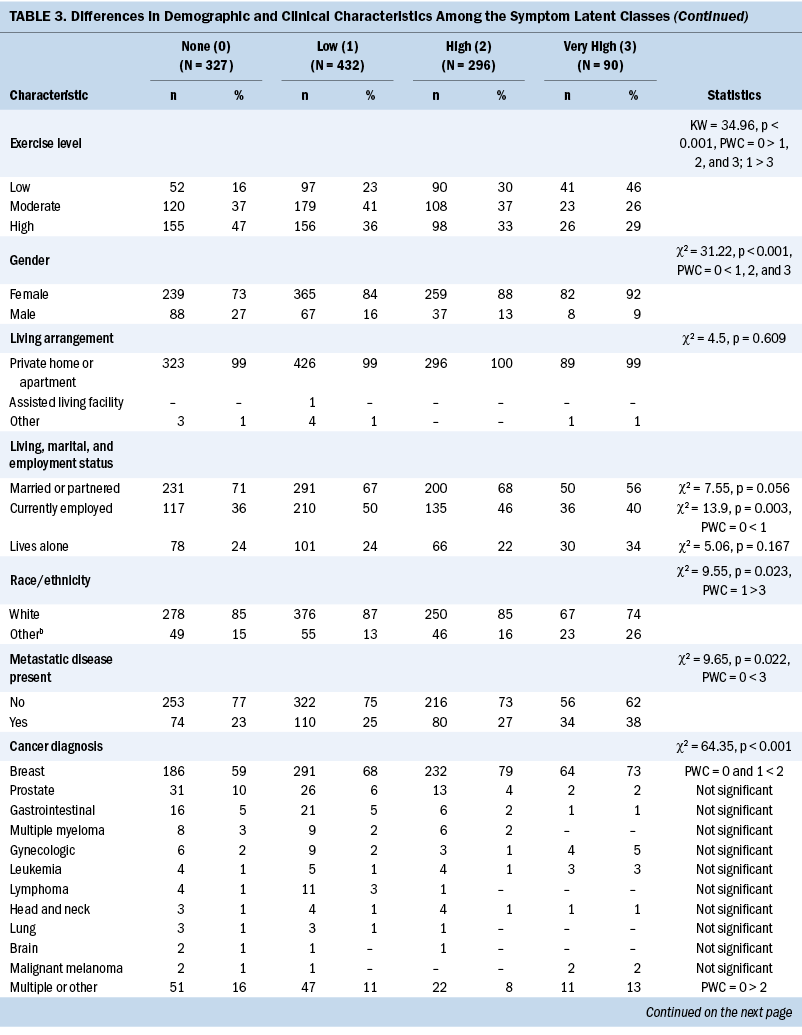
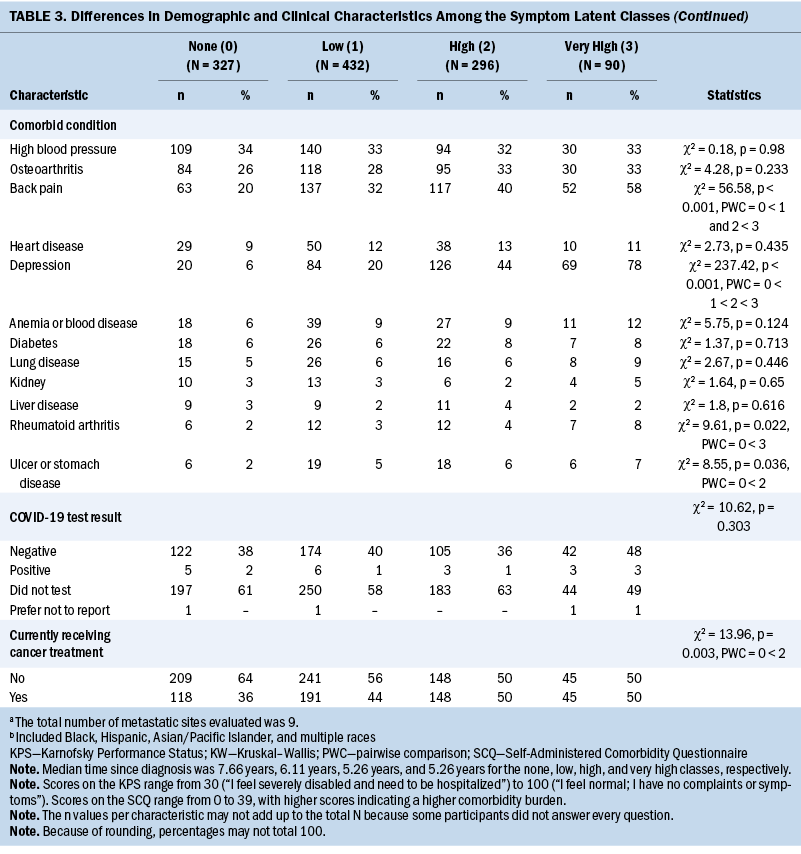
Stress and Resilience
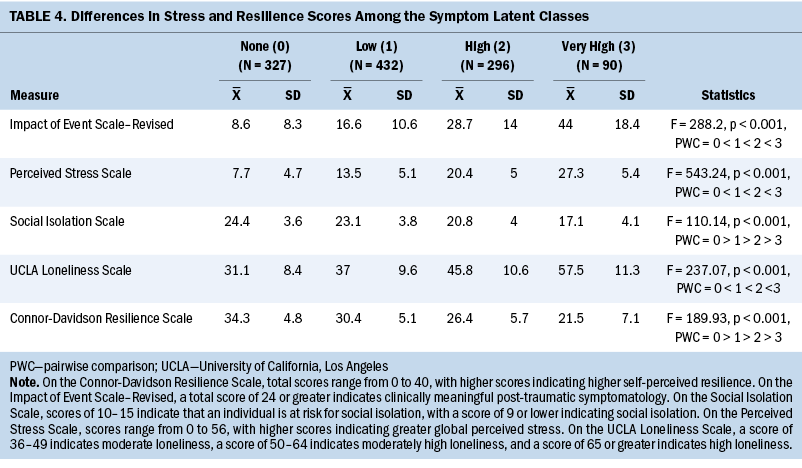
Differences among the four classes in IES-R, PSS, and UCLA Loneliness Scale scores followed similar patterns (see Table 4). Differences among the classes in Social Isolation Scale and Connor-Davidson Resilience Scale scores also followed similar patterns.
Discussion
This study is the first to use LCPA to evaluate for distinct symptom profiles using nine common symptoms as reported by patients with cancer during the height of the COVID-19 pandemic when vaccines and antiviral medications were not available. These results can be used as benchmark data of the symptom burden of patients with cancer for comparative purposes following the broad availability of vaccines and the lifting of social distancing and shelter-in-place orders, as well as for the evaluation of patients with cancer with long-term effects of COVID-19 (Mafi et al., 2022; Montani et al., 2022). Of note, compared to previous studies that found three (Doong et al., 2015; Hammer et al., 2022) to four (Miaskowski et al., 2006; Pud et al., 2008) distinct symptom profiles using the prespecified symptom cluster of pain, fatigue, sleep disturbance, and depression, the current study identified four distinct symptom profiles. Although none of the previous studies identified a very high class (8% of the current sample), all of them found a high class whose sizes were relatively consistent with the current study (e.g., 6.6% [Pud et al., 2008], 7.1% [Doong et al., 2015], 10.8% [Hammer et al., 2022], and 15% [Miaskowski et al., 2006]). These consistent results suggest that about 10% of patients with cancer experience a high to very high symptom burden.
Although in the current study the classes were named based on the distribution of clinically meaningful cutoff scores across the nine symptoms, it is notable that 67% of symptoms in the low class and 100% of symptoms in the high and very high classes exceeded these cutoff scores. The following sections compare the current study’s results regarding symptom severity and modifiable and nonmodifiable risk factors to extant literature prior to and during the COVID-19 pandemic.
Symptoms
Regarding depression and state anxiety, 34% of the sample (high and very high classes) reported clinically meaningful levels of both symptoms. These prevalence rates are consistent with a systematic review and meta-analysis, which found that during the COVID-19 pandemic, overall prevalence rates for depression and anxiety in the general population were 33.7% and 31.9%, respectively (Salari et al., 2020). Although the Center for Epidemiological Studies–Depression scale scores for the two highest classes (23.3 and 37.6) were comparable to outpatients receiving chemotherapy (Oppegaard et al., 2022), they were significantly higher than scores for survivors with (13.5) and without (6.7) chemotherapy-induced peripheral neuropathy (CIPN) (Miaskowski et al., 2018) obtained prior to the COVID-19 pandemic. A similar pattern was observed for state anxiety scores in the two highest classes (45.9 and 61.3), which were comparable to scores for outpatients receiving chemotherapy (Oppegaard et al., 2021) but higher than scores for survivors with (35.5) and without (28.4) CIPN (Miaskowski et al., 2018). Of note, consistent with a systematic review that showed that having a stable high monthly income was a protective factor for depression and anxiety in older adults during the pandemic (Ciuffreda et al., 2021), patients in the none class had a higher annual household income and lower rates of depression than patients in the very high class (6% versus 78%, respectively).
In terms of cognitive function, 72% of the sample reported moderate to high levels of cognitive impairment. This percentage is slightly higher than the 43%–66.6% reported in a systematic review of cognitive impairment in patients who were hospitalized for the COVID-19 virus (Alnefeesi et al., 2021). However, it is comparable to the 75% occurrence rate reported for patients with cancer prior to the pandemic (Janelsins et al., 2014). Regarding Attentional Function Index scores for the two highest classes (5.4 and 3.5), these scores are comparable to scores reported by outpatients receiving chemotherapy (Atallah et al., 2020) but significantly lower than scores for survivors with (6.2) and without (7.5) CIPN (Miaskowski et al., 2018).
Similar to the 60.7% prevalence rate for sleep problems in patients with cancer reported in a meta-analysis (Al Maqbali et al., 2022), 72% of the current sample reported clinically meaningful levels of sleep disturbance. However, in a study that compared breast cancer survivors to healthy women during the COVID-19 pandemic (Bethea et al., 2022), only 10% and 13.5% reported sleep disturbances, respectively. One potential reason for these disparate findings is that in the study of breast cancer survivors (Bethea et al., 2022), sleep disturbance was evaluated using a single item from the Center for Epidemiological Studies–Depression scale rather than a multidimensional sleep disturbance measure like the GSDS. Equally important, GSDS scores for the two highest classes were significantly higher than scores reported by survivors with (51.4) and without (39.2) CIPN (Miaskowski et al., 2018).
Similar to sleep disturbance—and most likely linked with this symptom—72% of patients reported clinically meaningful levels of morning fatigue and decrements in morning and evening energy. In addition, the high and very high classes had clinically meaningful levels of evening fatigue. These results are consistent with previous studies that demonstrated positive associations between evening fatigue (Wright et al., 2017) and decrements in energy (Abid et al., 2017) and sleep disturbance in patients receiving chemotherapy prior to the COVID-19 pandemic. Compared to cancer survivors with and without CIPN, morning (3.5 and 2.5) and evening (5.4 and 5.3) fatigue was significantly higher in the current sample. In addition, decrements in morning (4.4 and 5.4) and evening (3.4 and 4.1) energy were worse in the current sample compared to survivors with and without CIPN (Miaskowski et al., 2018).
Moderate to severe pain was reported by all four classes (100% of patients). Of note, this prevalence rate is significantly higher than the 38% reported in a meta-analysis that considered a pain rating of 5 or greater to equate with moderate or severe pain in patients with cancer (van den Beuken-van Everdingen et al., 2016). Although the specific causes of pain in the current sample were not assessed, the prevalence of osteoarthritis ranged from 26% to 33%, and the prevalence of back pain ranged from 20% to 58%. As noted by Paice (2022), managing pain in patients with cancer during the opioid epidemic and the COVID-19 pandemic posed significant challenges. Although trying to mitigate opioid misuse, shelter-in-place orders severely limited patients’ abilities to access nonpharmacologic interventions (e.g., physical therapy, acupuncture) and mental health services, which may have contributed to exacerbations in pain.
Demographic and Clinical Risk Factors
As shown in Table 5, compared to the none class, several common and distinct risk factors were associated with a higher symptom burden. Consistent with previous reports of patients with cancer, younger age (Doong et al., 2015; Hammer et al., 2022; Miaskowski et al., 2006) and being female (Hammer et al., 2022; Miaskowski et al., 2014) were associated with a higher symptom burden. Age-related differences in symptom burden are often attributed to a response shift in older adults’ ability to adapt to their changing health status (Sprangers & Schwartz, 1999). In the context of the loneliness and social isolation associated with the COVID-19 pandemic, older patients with cancer, who may be more accustomed to a homebound lifestyle, may experience less disruption in their daily routines than younger adults of working age and an associated decrease in symptoms (Clifton et al., 2022). In terms of gender differences, the results may be influenced by the high percentage of patients with breast cancer in the current sample and warrant additional investigation.
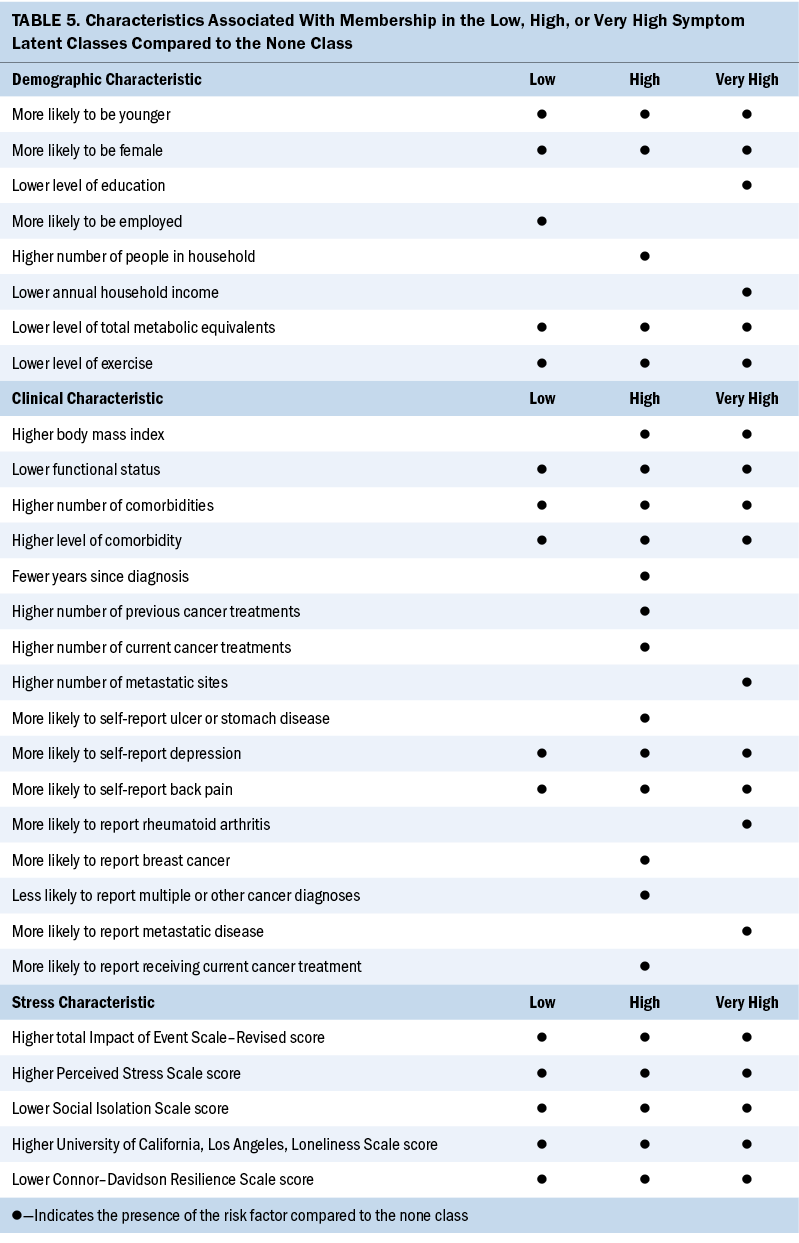
Compared to the other three classes, in addition to younger age and female sex, patients in the very high class had a lower level of education, were more likely to identify as non-White, and had a lower annual household income. Although associations between these characteristics and a higher symptom burden were reported in previous studies of patients with cancer (Hammer et al., 2022; Miaskowski et al., 2014), in the context of the inequities in health care unearthed during the COVID-19 pandemic (Boserup et al., 2020; Hawkins et al., 2020; Llanos et al., 2023), increased attention needs to be paid to these social determinants of health in cancer care. Clinicians can assess for associations between a higher symptom burden and these social determinants of health, as well as those that were not assessed in this study (e.g., food insecurity, discrimination).
A higher BMI and comorbidity burden and lower level of exercise and functional status were common clinical characteristics associated with membership in the high and very high classes. Of note, patients in the two highest classes had BMIs in the overweight and obese categories (Centers for Disease Control and Prevention, 2022). Although the pre–COVID-19 pandemic weight of the patients is unknown, the results of a systematic review suggest that during the first year of the pandemic, children and adults incurred potentially clinically significant increases in weight and BMI (Anderson et al., 2023). Equally important, the extremely low level of exercise in the two highest symptom burden classes may have existed or were exacerbated by mitigation procedures because of the COVID-19 pandemic (Hilbold et al., 2023). Because exercise is beneficial in decreasing symptom burden in patients with cancer (Larson et al., 2023; Matthews et al., 2018; Zhang et al., 2023; Zhu et al., 2022), clinicians can assess for post–COVID-19 pandemic weight gain and changes in exercise behaviors, counsel patients to start or maintain a regular exercise routine, and make appropriate referrals to enhance their functional status.
A dose–response effect was seen among the symptom classes in the number of comorbidities and comorbidity burden. This linkage between a higher symptom burden and higher comorbidity burden was highlighted in a review by George et al. (2021). George et al. (2021) noted that 37.9%–74.3% of patients with colorectal cancer and 12.6%–49% of patients with breast cancer have at least one comorbidity. In addition, the presence of comorbidities was associated with less optimal cancer treatment and associated decreases in survival. Therefore, oncology clinicians need to work collaboratively with patients’ primary care providers to effectively manage these conditions and associated symptoms.
Stress and Resilience
All the stress and resilience measures exhibited a dose–response effect (i.e., as symptom burden increased, cancer- and COVID-19–specific stress, global stress, and loneliness scores increased, and social isolation and resilience scores decreased). Although not linked definitively to a higher symptom burden, as noted by Aknin et al. (2022), psychological distress increased in the general population during the early months of the COVID-19 pandemic. In addition, psychological distress was particularly pronounced among individuals who were female, were young, and had children aged younger than 5 years.
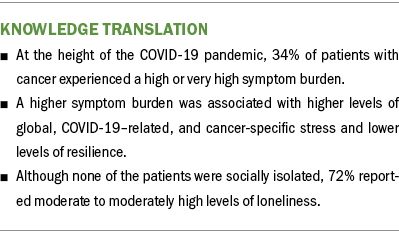
Although evidence suggests that the prevalence of post-traumatic stress disorder in the general population during the COVID-19 pandemic was about 22% (Cénat et al., 2021), 34% of patients in the high and very high classes had total IES-R scores that were suggestive of partial or probable post-traumatic stress disorder. This measure specifically assessed cancer- and COVID-19–related stress. In addition, this same percentage of patients had global stress scores on the PSS that exceeded normative scores for the U.S. population in 2006 (mean = 12.73, SD = 7.34) and during the 2009 economic downturn (mean = 15.21, SD = 7.28) (Cohen & Janicki-Deverts, 2012). These high levels of both types of stress may be related to the concerns of patients with cancer about being at increased risk for more severe disease and complications, as well as increased mortality, if they contracted the COVID-19 virus (Colomer-Lahiguera et al., 2021; Dhada et al., 2021; Jammu et al., 2021). Although increased stress is associated with a higher symptom burden in patients with cancer (Langford et al., 2022; Shin et al., 2022; Stacker et al., 2023), the current study’s results suggest that the added stress associated with the pandemic markedly increased the severity of nine common symptoms in 34% of patients.
Social isolation and loneliness can be considered somewhat unique types of stress from the COVID-19 pandemic (Harden et al., 2020; Killgore et al., 2020; Pietrabissa & Simpson, 2020). Although none of the classes had Social Isolation Scale scores indicative of being socially isolated, 72% of the sample had moderate to moderately high scores on the UCLA Loneliness Scale. The absence of a correlation between social isolation and loneliness is consistent with a population-based study of older adults in their last years of life (Kotwal et al., 2021), which found that 19% of older adults experienced social isolation, 18% experienced loneliness, and only 5% experienced both types of stress. However, the occurrence rate for loneliness in the current sample is higher than the 28% reported in a review of studies of older adults during the COVID-19 pandemic (Su et al., 2023). Most of the research on the impact of loneliness on symptom burden during the COVID-19 pandemic focused on mental health problems (Aknin et al., 2022; Giacco, 2023; Jamil et al., 2022). However, the current study’s results suggest that these added types of stress were associated with clinically meaningful levels of physical and psychological symptoms in more than one-third of patients.
Research on associations between symptom burden and resilience in patients with cancer has focused primarily on psychological symptoms (Aizpurua-Perez & Perez-Tejada, 2020; Oppegaard et al., 2021; Shin et al., 2022; Tamura, 2021; Tamura et al., 2021) and quality-of-life outcomes (FranjiĆ et al., 2021; Macía et al., 2020; Zhang et al., 2017). In general, patients with cancer with higher levels of resilience report lower levels of psychological distress (Aizpurua-Perez & Perez-Tejada, 2020; Oppegaard et al., 2021; Shin et al., 2022; Tamura, 2021; Tamura et al., 2021) and better quality of life (FranjiĆ et al., 2021; Macía et al., 2020; Zhang et al., 2017). Although no studies were identified that evaluated associations between symptom burden in patients with cancer and their levels of resilience during the COVID-19 pandemic, resilience scores in the three classes with the highest symptom burden were below the normative score for the general population of the United States (Campbell-Sills et al., 2009). Given that a variety of interventions (e.g., cognitive behavioral therapy, mindfulness training) are known to enhance resilience (Joyce et al., 2018; Ludolph et al., 2019), clinicians can refer patients for these types of interventions and assess their efficacy in reducing symptom burden, particularly in high-risk patients.
Limitations
Several limitations warrant consideration. Given that a majority of patients were women with breast cancer, the results may not generalize to men or patients with other types of cancer. In this study, the patients were well educated and had a household income greater than the median household income of $70,784 as reported by the U.S. Census Bureau in 2021. Therefore, the findings may not be generalizable to patients with lower levels of education or of a lower socioeconomic status. Because patients were recruited through an online survey, sampling bias may be present, skewing the sample to individuals who were technology literate and had access to email and the internet. Given the study’s cross-sectional design, causal relationships between symptom burden and various risk factors cannot be determined.
Implications for Nursing
Despite this study’s limitations, many of the risk factors associated with a higher symptom burden profile are amenable to interventions. For example, weight management and exercise interventions can be prescribed to decrease many of the common symptoms associated with cancer and its treatments (Larson et al., 2023; Matthews et al., 2018; Zhang et al., 2023; Zhu et al., 2022). In addition, stress-reduction interventions and resilience training, along with targeted pharmacologic interventions, can be prescribed to decrease the extremely high symptom burden of these high-risk patients.
Conclusion
Findings from this study suggest that during the height of the COVID-19 pandemic, 34% of patients with cancer experienced a high or very high symptom burden. Data presented in this article can be used as benchmark data to assess the long-term impact of COVID-19 on patients’ symptom experiences. One major unanswered question is whether the symptom burden of patients with cancer has decreased postpandemic. Future research administering the same measures used in this study can use this study’s results as benchmark data for comparative purposes and for the development of individualized symptom management interventions.
About the Authors
Ji Hun Kwak, RN, BA, is a master’s student, Lynda A. Mackin, PhD, AGPCNP-BC®, CCNS, GS-C, is a health science clinical professor, Astrid Block, RN, MS, CNS, is an associate clinical professor, Sueann Mark, MS, PhD, RN, AOCNS®, is an assistant clinical professor, Steven M. Paul, PhD, is a data analyst, and Bruce A. Cooper, PhD, is a research data analyst III in the Department of Physiological Nursing, all in the School of Nursing at the University of California, San Francisco; Maura Abbott, PhD, AOCNP®, CPNP-PC, AC, is the assistant dean of clinical affairs, the coordinator of the oncology subspecialty, and an associate professor of nursing in the School of Nursing, all at the Columbia University Irving Medical Center in New York, NY; Susan M. Chang, MD, is a professor in the Department of Neurological Surgery at the University of California, San Francisco; Marilyn J. Hammer, PhD, DC, RN, FAAN, is the director of the Phyllis F. Cantor Center for Research in Nursing and Patient Care Services at Dana-Farber Cancer Institute in Boston, MA; Kord M. Kober, PhD, is an associate professor in the School of Nursing and Jon D. Levine, MD, PhD, is a professor in the Department of Medicine, both at the University of California, San Francisco; Rachel Pozzar, PhD, RN, is an instructor at Dana-Farber Cancer Institute; and Kim F. Rhoads, MD, MS, MPH, is the associate director of the Helen Diller Family Comprehensive Cancer Center, Karin E. Snowberg, MA, is a project director, Katy K. Tsai, MD, is an assistant clinical professor in the Division of Hematology and Oncology in the Department of Medicine, Erin L. Van Blarigan, ScD, is an associate professor of epidemiology and biostatistics in the Department of Urology, Katherine Van Loon, MD, MPH, is an associate professor of clinical medicine in the School of Medicine, and Christine Miaskowski, RN, PhD, is a professor in the Department of Physiological Nursing in the School of Nursing, all at the University of California, San Francisco. No financial relationships to disclose. Kwak, Chang, Hammer, Pozzar, Snowberg, Van Loon, and Miaskowski contributed to the conceptualization and design. Hammer, Pozzar, Snowberg, and Miaskowski completed the data collection. Paul, Cooper, and Miaskowski provided statistical support. Kwak and Miaskowski provided the analysis. Kwak, Mackin, Block, Mark, Abbott, Chang, Hammer, Kober, Levine, Pozzar, Rhoads, Snowberg, Tsai, Van Blarigan, Van Loon, and Miaskowski contributed to the manuscript preparation. Miaskowski can be reached at chris.miaskowski@ucsf.edu, with copy to ONFEditor@ons.org. (Submitted April 2023. Accepted May 24, 2023.)
References
Aapro, M., Lyman, G.H., Bokemeyer, C., Rapoport, B.L., Mathieson, N., Koptelova, N., . . . Kuderer, N.M. (2021). Supportive care in patients with cancer during the COVID-19 pandemic. ESMO Open, 6(1), 100038. https://doi.org/10.1016/j.esmoop.2020.100038
Abid, H., Kober, K.M., Smoot, B., Paul, S.M., Hammer, M., Levine, J.D., . . . Miaskowski, C. (2017). Common and distinct characteristics associated with trajectories of morning and evening energy in oncology patients receiving chemotherapy. Journal of Pain and Symptom Management, 53(5), 887–900.e2. https://doi.org/10.1016/j.jpainsymman.2016.12.339
Aizpurua-Perez, I., & Perez-Tejada, J. (2020). Resilience in women with breast cancer: A systematic review. European Journal of Oncology Nursing, 49, 101854. https://doi.org/10.1016/j.ejon.2020.101854
Aknin, L.B., De Neve, J.-E., Dunn, E.W., Fancourt, D.E., Goldberg, E., Helliwell, J.F., . . . Ben Amor, Y. (2022). Mental health during the first year of the COVID-19 pandemic: A review and recommendations for moving forward. Perspectives on Psychological Science, 17(4), 915–936. https://doi.org/10.1177/17456916211029964
Allemann-Su, Y.-Y., Vetter, M., Koechlin, H., Conley, Y., Paul, S.M., Cooper, B.A., . . . Katapodi, M.C. (2023). Distinct cognitive function profiles are associated with a higher presurgery symptom burden in patients with breast cancer. Cancer Nursing, 46(4), E208–E217. https://doi.org/10.1097/NCC.0000000000001114
Al Maqbali, M., Al Sinani, M., Alsayed, A., & Gleason, A.M. (2022). Prevalence of sleep disturbance in patients with cancer: A systematic review and meta-analysis. Clinical Nursing Research, 31(6), 1107–1123. https://doi.org/10.1177/10547738221092146
Alnefeesi, Y., Siegel, A., Lui, L.M.W., Teopiz, K.M., Ho, R.C.M., Lee, Y., . . . McIntyre, R.S. (2021). Impact of SARS-CoV-2 infection on cognitive function: A systematic review. Frontiers in Psychiatry, 11, 621773. https://doi.org/10.3389/fpsyt.2020.621773
Anderson, L.N., Yoshida-Montezuma, Y., Dewart, N., Jalil, E., Khattar, J., De Rubeis, V., . . . Mbuagbaw, L. (2023). Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obesity Reviews, 24(5), e13550. https://doi.org/10.1111/obr.13550
Ashbury, F.D. (2021). COVID-19 and supportive cancer care: Key issues and opportunities. Current Opinion in Oncology, 33(4), 295–300. https://doi.org/10.1097/CCO.0000000000000729
Atallah, M., Cooper, B., Muñoz, R.F., Paul, S.M., Anguera, J., Levine, J.D., . . . Dunn, L.B. (2020). Psychological symptoms and stress are associated with decrements in attentional function in cancer patients undergoing chemotherapy. Cancer Nursing, 43(5), 402–410. https://doi.org/10.1097/NCC.0000000000000713
Bethea, T.N., Zhai, W., Zhou, X., Ahles, T.A., Ahn, J., Cohen, H.J., . . . Carroll, J.E. (2022). Associations between longitudinal changes in sleep disturbance and depressive and anxiety symptoms during the COVID-19 virus pandemic among older women with and without breast cancer in the thinking and living with breast cancer study. Cancer Medicine, 11(17), 3352–3363. https://doi.org/10.1002/cam4.4682
Boserup, B., McKenney, M., & Elkbuli, A. (2020). Disproportionate impact of COVID-19 pandemic on racial and ethnic minorities. American Surgeon, 86(12), 1615–1622. https://doi.org/10.1177/0003134820973356
Calvo-Schimmel, A., Paul, S.M., Cooper, B.A., Shin, J., Harris, C., Oppegaard, K., . . . Miaskowski, C. (2022). Oncology outpatients with worse anxiety and sleep disturbance profiles are at increased risk for a higher symptom burden and poorer quality of life. Cancer Nursing. Advance online publication. https://doi.org/10.1097/NCC.0000000000001139
Campbell-Sills, L., Forde, D.R., & Stein, M.B. (2009). Demographic and childhood environmental predictors of resilience in a community sample. Journal of Psychiatric Research, 43(12), 1007–1012. https://doi.org/10.1016/j.jpsychires.2009.01.013
Campbell-Sills, L., & Stein, M.B. (2007). Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20(6), 1019–1028. https://doi.org/10.1002/jts.20271
Celeux, G., & Soromenho, G. (1996). An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification, 13(2), 195–212. https://doi.org/10.1007/BF01246098
Cénat, J.M., Blais-Rochette, C., Kokou-Kpolou, C.K., Noorishad, P.-G., Mukunzi, J.N., McIntee, S.-E., . . . Labelle, P.R. (2021). Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Research, 295, 113599. https://doi.org/10.1016/j.psychres.2020.113599
Centers for Disease Control and Prevention. (2022). About adult BMI. U.S. Department of Health and Human Services. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi
Cimprich, B., So, H., Ronis, D.L., & Trask, C. (2005). Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psycho-Oncology, 14(1), 70–78. https://doi.org/10.1002/pon.821
Cimprich, B., Visovatti, M., & Ronis, D.L. (2011). The Attentional Function Index—A self-report cognitive measure. Psycho-Oncology, 20(2), 194–202. https://doi.org/10.1002/pon.1729
Ciuffreda, G., Cabanillas-Barea, S., Carrasco-Uribarren, A., Albarova-Corral, M.I., Argüello-Espinosa, M.I., & Marcén-Román, Y. (2021). Factors associated with depression and anxiety in adults ≥60 years old during the COVID-19 pandemic: A systematic review. International Journal of Environmental Research and Public Health, 18(22), 11859. https://doi.org/10.3390/ijerph182211859
Clifton, K., Gao, F., Jabbari, J., Van Aman, M., Dulle, P., Hanson, J., & Wildes, T.M. (2022). Loneliness, social isolation, and social support in older adults with active cancer during the COVID-19 pandemic. Journal of Geriatric Oncology, 13(8), 1122–1131. https://doi.org/10.1016/j.jgo.2022.08.003
Cohen, S., & Janicki-Deverts, D. (2012). Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. Journal of Applied Social Psychology, 42(6), 1320–1334. https://doi.org/10.1111/j.1559-1816.2012.00900.x
Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://www.ncbi.nlm.nih.gov/pubmed/6668417
Colomer-Lahiguera, S., Ribi, K., Dunnack, H.J., Cooley, M.E., Hammer, M.J., Miaskowski, C., & Eicher, M. (2021). Experiences of people affected by cancer during the outbreak of the COVID-19 pandemic: An exploratory qualitative analysis of public online forums. Supportive Care in Cancer, 29(9), 4979–4985. https://doi.org/10.1007/s00520-021-06041-y
Craig, C.L., Marshall, A.L., Sjöström, M., Bauman, A.E., Booth, M.L., Ainsworth, B.E., . . . Oja, P. (2003). International Physical Activity Questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35(8), 1381–1395. https://doi.org/10.1249/01.MSS.0000078924.61453.FB
Creamer, M., Bell, R., & Failla, S. (2003). Psychometric properties of the Impact of Event Scale–Revised. Behaviour Research and Therapy, 41(12), 1489–1496. https://doi.org/10.1016/j.brat.2003.07.010
Daut, R.L., Cleeland, C.S., & Flanery, R.C. (1983). Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain, 17(2), 197–210. https://doi.org/10.1016/0304-3959(83)90143-4
Dhada, S., Stewart, D., Cheema, E., Hadi, M.A., & Paudyal, V. (2021). Cancer services during the COVID-19 pandemic: Systematic review of patient’s and caregiver’s experiences. Cancer Management and Research, 13, 5875–5887. https://doi.org/10.2147/CMAR.S318115
Doong, S.-H., Dhruva, A., Dunn, L.B., West, C., Paul, S.M., Cooper, B.A., . . . Miaskowski, C. (2015). Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biological Research for Nursing, 17(3), 237–247. https://doi.org/10.1177/1099800414550394
Fletcher, B.S., Paul, S.M., Dodd, M.J., Schumacher, K., West, C., Cooper, B., . . . Miaskowski, C.A. (2008). Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology, 26(4), 599–605. https://doi.org/10.1200/JCO.2007.12.2838
Franjić, D., Babić, D., Marijanović, I., & Martinac, M. (2021). Association between resilience and quality of life in patients with colon cancer. Psychiatria Danubina, 33(Suppl. 13), 297–303.
Gallagher, S., Bennett, K.M., & Roper, L. (2021). Loneliness and depression in patients with cancer during COVID-19. Journal of Psychosocial Oncology, 39(3), 445–451. https://doi.org/10.1080/07347332.2020.1853653
George, M., Smith, A., Sabesan, S., & Ranmuthugala, G. (2021). Physical comorbidities and their relationship with cancer treatment and its outcomes in older adult populations: Systematic review. JMIR Cancer, 7(4), e26425. https://doi.org/10.2196/26425
Giacco, D. (2023). Loneliness and mood disorders: Consequence, cause and/or unholy alliance? Current Opinion in Psychiatry, 36(1), 47–53. https://doi.org/10.1097/YCO.0000000000000832
Hallal, P.C., & Victora, C.G. (2004). Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Medicine and Science in Sports and Exercise, 36(3), 556. https://doi.org/10.1249/01.mss.0000117161.66394.07
Hammer, M.J., Cooper, B., Paul, S.M., Kober, K.M., Cartwright, F., Conley, Y.P., . . . Miaskowski, C. (2022). Identification of distinct symptom profiles in cancer patients using a pre-specified symptom cluster. Journal of Pain and Symptom Management, 64(1), 17–27. https://doi.org/10.1016/j.jpainsymman.2022.03.007
Harden, K., Price, D.M., Mason, H., & Bigelow, A. (2020). COVID-19 shines a spotlight on the age-old problem of social isolation. Journal of Hospice and Palliative Nursing, 22(6), 435–441. https://doi.org/10.1097/NJH.0000000000000693
Hawkins, R.B., Charles, E.J., & Mehaffey, J.H. (2020). Socio-economic status and COVID-19-related cases and fatalities. Public Health, 189, 129–134. https://doi.org/10.1016/j.puhe.2020.09.016
Hilbold, E., Bär, C., & Thum, T. (2023). COVID-19: Insights into long-term manifestations and lockdown impacts. Journal of Sport and Health Science, 12(4), 438–463. https://doi.org/10.1016/j.jshs.2023.02.006
Huang, V., Mackin, L., Kober, K.M., Paul, S.M., Cooper, B.A., Conley, Y.P., . . . Miaskowski, C. (2022). Distinct sleep disturbance and cognitive dysfunction profiles in oncology outpatients receiving chemotherapy. Supportive Care in Cancer, 30(11), 9243–9254. https://doi.org/10.1007/s00520-022-07350-6
Jamil, A., Syed, J., Kanwal, S., Ain, Q.U., Namroz, N., Gul, A., & Jamil, A. (2022). Loneliness and mental health related impacts of COVID-19: A narrative review. International Journal of Adolescent Medicine and Health, 35(1), 21–30. https://doi.org/10.1515/ijamh-2022-0032
Jammu, A.S., Chasen, M.R., Lofters, A.K., & Bhargava, R. (2021). Systematic rapid living review of the impact of the COVID-19 pandemic on cancer survivors: Update to August 27, 2020. Supportive Care in Cancer, 29(6), 2841–2850. https://doi.org/10.1007/s00520-020-05908-w
Janelsins, M.C., Kesler, S.R., Ahles, T.A., & Morrow, G.R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry, 26(1), 102–113. https://doi.org/10.3109/09540261.2013.864260
Joyce, S., Shand, F., Tighe, J., Laurent, S.J., Bryant, R.A., & Harvey, S.B. (2018). Road to resilience: A systematic review and meta-analysis of resilience training programmes and interventions. BMJ Open, 8(6), e017858. https://doi.org/10.1136/bmjopen-2017-017858
Karnofsky, D. (1977). Performance scale. Plenum Press.
Killgore, W.D.S., Cloonan, S.A., Taylor, E.C., Lucas, D.A., & Dailey, N.S. (2020). Loneliness during the first half-year of COVID-19 lockdowns. Psychiatry Research, 294, 113551. https://doi.org/10.1016/j.psychres.2020.113551
Knight, R.G., Chisholm, B.J., Marsh, N.V., & Godfrey, H.P. (1988). Some normative, reliability, and factor analytic data for the revised UCLA Loneliness Scale. Journal of Clinical Psychology, 44(2), 203–206. https://doi.org/10.1002/1097-4679(198803)44:2<203::aid-jclp2270440218>3…
Kotwal, A.A., Cenzer, I.S., Waite, L.J., Covinsky, K.E., Perissinotto, C.M., Boscardin, W.J., . . . Smith, A.K. (2021). The epidemiology of social isolation and loneliness among older adults during the last years of life. Journal of the American Geriatrics Society, 69(11), 3081–3091. https://doi.org/10.1111/jgs.17366
Langford, D.J., Eaton, L., Kober, K.M., Paul, S.M., Cooper, B.A., Hammer, M.J., . . . Miaskowski, C. (2022). A high stress profile is associated with severe pain in oncology patients receiving chemotherapy. European Journal of Oncology Nursing, 58, 102135. https://doi.org/10.1016/j.ejon.2022.102135
Lanza, S.T., Flaherty, B.P., & Collins, L.M. (2003). Latent class and latent transition analysis. In J.A. Schinka & W.F. Velicer (Eds.), Handbook of psychology: Vol. 2. Research methods in psychology (pp. 663–685). John Wiley and Sons. https://doi.org/10.1002/0471264385.wei0226
Larson, E.A., Dalamaga, M., & Magkos, F. (2023). The role of exercise in obesity-related cancers: Current evidence and biological mechanisms. Seminars in Cancer Biology, 91, 16–26. https://doi.org/10.1016/j.semcancer.2023.02.008
Lee, K.A., Hicks, G., & Nino-Murcia, G. (1991). Validity and reliability of a scale to assess fatigue. Psychiatry Research, 36(3), 291–298. https://doi.org/10.1016/0165-1781(91)90027-m
Lin, Y., Bailey, D.E., Xiao, C., Hammer, M., Paul, S.M., Cooper, B.A., . . . Miaskowski, C. (2022). Distinct co-occurring morning and evening fatigue profiles in patients with gastrointestinal cancers receiving chemotherapy. Cancer Nursing. Advance online publication. https://doi.org/10.1097/NCC.0000000000001148
Llanos, A.A.M., Ashrafi, A., Ghosh, N., Tsui, J., Lin, Y., Fong, A.J., . . . Heckman, C.J. (2023). Evaluation of inequities in cancer treatment delay or discontinuation following SARS-CoV-2 infection. JAMA Network Open, 6(1), e2251165. https://doi.org/10.1001/jamanetworkopen.2022.51165
Ludolph, P., Kunzler, A.M., Stoffers-Winterling, J., Helmreich, I., & Lieb, K. (2019). Interventions to promote resilience in cancer patients. Deutsches Arzteblatt International, 51-52, 865–872. https://doi.org/10.3238/arztebl.2019.0865
Macía, P., Barranco, M., Gorbeña, S., & Iraurgi, I. (2020). Expression of resilience, coping and quality of life in people with cancer. PLOS ONE, 15(7), e0236572. https://doi.org/10.1371/journal.pone.0236572
Mafi, A.R., Ghanbari Motlagh, A., & Azadeh, P. (2022). The impact of COVID-19 on cancer recurrence: A narrative review. Archives of Iranian Medicine, 25(7), 450–455. https://doi.org/10.34172/aim.2022.74
Matthews, E.E., Janssen, D.W., Djalilova, D.M., & Berger, A.M. (2018). Effects of exercise on sleep in women with breast cancer: A systematic review. Sleep Medicine Clinics, 13(3), 395–417. https://doi.org/10.1016/j.jsmc.2018.04.007
Mauri, D., Kamposioras, K., Tolia, M., Alongi, F., & Tzachanis, D. (2020). Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncology, 21(6), 759–760. https://doi.org/10.1016/S1470-2045(20)30278-3
Mazor, M., Paul, S.M., Chesney, M.A., Chen, L.-M., Smoot, B., Topp, K., . . . Miaskowski, C. (2019). Perceived stress is associated with a higher symptom burden in cancer survivors. Cancer, 125(24), 4509–4515. https://doi.org/10.1002/cncr.32477
Miaskowski, C., Cooper, B.A., Melisko, M., Chen, L.-M., Mastick, J., West, C., . . . Aouizerat, B.E. (2014). Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer, 120(15), 2371–2378. https://doi.org/10.1002/cncr.28699
Miaskowski, C., Cooper, B.A., Paul, S.M., Dodd, M., Lee, K., Aouizerat, B.E., . . . Bank, A. (2006). Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncology Nursing Forum, 33(5), E79–E89. https://doi.org/10.1188/06.ONF.E79-E89
Miaskowski, C., Paul, S.M., Mastick, J., Abrams, G., Topp, K., Smoot, B., . . . Levine, J.D. (2018). Associations between perceived stress and chemotherapy-induced peripheral neuropathy and otoxicity in adult cancer survivors. Journal of Pain and Symptom Management, 56(1), 88–97. https://doi.org/10.1016/j.jpainsymman.2018.02.021
Miaskowski, C., Paul, S.M., Snowberg, K., Abbott, M., Borno, H., Chang, S., . . . Van Loon, K. (2020). Stress and symptom burden in oncology patients during the COVID-19 pandemic. Journal of Pain and Symptom Management, 60(5), e25–e34. https://doi.org/10.1016/j.jpainsymman.2020.08.037
Montani, D., Savale, L., Noel, N., Meyrignac, O., Colle, R., Gasnier, M., . . . Monnet, X. (2022). Post-acute COVID-19 syndrome. European Respiratory Review, 31(163), 210185. https://doi.org/10.1183/16000617.0185-2021
Moraliyage, H., De Silva, D., Ranasinghe, W., Adikari, A., Alahakoon, D., Prasad, R., . . . Bolton, D. (2021). Cancer in lockdown: Impact of the COVID-19 pandemic on patients with cancer. Oncologist, 26(2), e342–e344. https://doi.org/10.1002/onco.13604
Morina, N., Ehring, T., & Priebe, S. (2013). Diagnostic utility of the Impact of Event Scale–Revised in two samples of survivors of war. PLOS ONE, 8(12), e83916. https://doi.org/10.1371/journal.pone.0083916
Muthén, B.O. (2002). Beyond SEM: General latent variable modeling. Behaviormetrika, 29(1), 81–117. https://www.statmodel.com/download/muthen1.pdf
Muthén, B., & Shedden, K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55(2), 463–469. https://doi.org/10.1111/j.0006-341x.1999.00463.x
Muthén, L.K., & Muthén, B.O. (1998–2010). Mplus user’s guide, version 6. https://socialwork.wayne.edu/research//pdf/mplus-users-guide.pdf
Nicholson, N.R., Feinn, R., Casey, E.A., & Dixon, J. (2020). Psychometric evaluation of the Social Isolation Scale in older adults. Gerontologist, 60(7), e491–e501. https://doi.org/10.1093/geront/gnz083
Nylund, K.L., Asparouhov, T., & Muthén, B.O. (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling, 14(4), 535–569. https://doi.org/10.1080/10705510701575396
Oppegaard, K., Harris, C.S., Shin, J., Paul, S.M., Cooper, B.A., Levine, J.D., . . . Miaskowski, C. (2021). Anxiety profiles are associated with stress, resilience and symptom severity in outpatients receiving chemotherapy. Supportive Care in Cancer, 29(12), 7825–7836. https://doi.org/10.1007/s00520-021-06372-w
Oppegaard, K., Shin, J., Harris, C.S., Schimmel, A., Paul, S.M., Cooper, B.A., . . . Miaskowski, C. (2022). Higher stress and symptom severity are associated with worse depressive symptom profiles in patients receiving chemotherapy. European Journal of Oncology Nursing, 58, 102031. https://doi.org/10.1016/j.ejon.2021.102031
Paice, J.A. (2022). Cancer pain during an epidemic and a pandemic. Current Opinion in Supportive and Palliative Care, 16(2), 55–59. https://doi.org/10.1097/SPC.0000000000000594
Pietrabissa, G., & Simpson, S.G. (2020). Psychological consequences of social isolation during COVID-19 outbreak. Frontiers in Psychology, 11, 2201. https://doi.org/10.3389/fpsyg.2020.02201
Pud, D., Ben Ami, S., Cooper, B.A., Aouizerat, B.E., Cohen, D., Radiano, R., . . . Miaskowski, C. (2008). The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. Journal of Pain and Symptom Management, 35(2), 162–170. https://doi.org/10.1016/j.jpainsymman.2007.03.010
Quinn-Scoggins, H.D., Cannings-John, R., Moriarty, Y., Whitelock, V., Whitaker, K.L., Grozeva, D., . . . Brain, K. (2021). Cancer symptom experience and help-seeking behaviour during the COVID-19 pandemic in the UK: A cross-sectional population survey. BMJ Open, 11(9), e053095. https://doi.org/10.1136/bmjopen-2021-053095
Radloff, L.S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. https://doi.org/10.1177/014662167700100306
Rentscher, K.E., Zhou, X., Small, B.J., Cohen, H.J., Dilawari, A.A., Patel, S.K., . . . Mandelblatt, J.S. (2021). Loneliness and mental health during the COVID-19 pandemic in older breast cancer survivors and noncancer controls. Cancer, 127(19), 3671–3679. https://doi.org/10.1002/cncr.33687
Russell, D., Peplau, L.A., & Cutrona, C.E. (1980). The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology, 39(3), 472–480. https://doi.org/10.1037//0022-3514.39.3.472
Russell, D., Peplau, L.A., & Ferguson, M.L. (1978). Developing a measure of loneliness. Journal of Personality Assessment, 42(3), 290–294. https://doi.org/10.1207/s15327752jpa4203_11
Russell, D.W. (1996). UCLA Loneliness Scale (version 3): Reliability, validity, and factor structure. Journal of Personality Assessment, 66(1), 20–40. https://doi.org/10.1207/s15327752jpa6601_2
Saini, K.S., Tagliamento, M., Lambertini, M., McNally, R., Romano, M., Leone, M., . . . de Azambuja, E. (2020). Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. European Journal of Cancer, 139, 43–50. https://doi.org/10.1016/j.ejca.2020.08.011
Salari, N., Hosseinian-Far, A., Jalali, R., Vaisi-Raygani, A., Rasoulpoor, S., Mohammadi, M., . . . Khaledi-Paveh, B. (2020). Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Globalization and Health, 16(1), 57. https://doi.org/10.1186/s12992-020-00589-w
Sangha, O., Stucki, G., Liang, M.H., Fossel, A.H., & Katz, J.N. (2003). The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism, 49(2), 156–163. https://doi.org/10.1002/art.10993
Shin, J., Harris, C., Oppegaard, K., Kober, K.M., Paul, S.M., Cooper, B.A., . . . Miaskowski, C. (2022). Worst pain severity profiles of oncology patients are associated with significant stress and multiple co-occurring symptoms. Journal of Pain, 23(1), 74–88. https://doi.org/10.1016/j.jpain.2021.07.001
Singh, S., Fletcher, G.G., Yao, X., & Sussman, J. (2021). Virtual care in patients with cancer: A systematic review. Current Oncology, 28(5), 3488–3506. https://doi.org/10.3390/curroncol28050301
Spielberger, C.D., Gorsuch, R.L., Lushene, R.E., Vagg, P.R., & Jacobs, G.A. (1983). Manual for the State-Trait Anxiety Inventory (Form Y1–Y2). Consulting Psychologists Press.
Sprangers, M.A., & Schwartz, C.E. (1999). The challenge of response shift for quality-of-life-based clinical oncology research. Annals of Oncology, 10(7), 747–749. https://doi.org/10.1023/a:1008305523548
Stacker, T., Kober, K.M., Dunn, L., Viele, C., Paul, S.M., Hammer, M.C., . . . Miaskowski, C. (2023). Associations between demographic, clinical, and symptom characteristics and stress in oncology patients receiving chemotherapy. Cancer Nursing, 46(1), E62–E69. https://doi.org/10.1097/NCC.0000000000001069
Su, Y., Rao, W., Li, M., Caron, G., D’Arcy, C., & Meng, X. (2023). Prevalence of loneliness and social isolation among older adults during the COVID-19 pandemic: A systematic review and meta-analysis. International Psychogeriatrics, 35(5), 229–241. https://doi.org/10.1017/S1041610222000199
Tamura, S. (2021). Factors related to resilience, anxiety/depression, and quality of life in patients with colorectal cancer undergoing chemotherapy in Japan. Asia-Pacific Journal of Oncology Nursing, 8(4), 393–402. https://doi.org/10.4103/apjon.apjon-2099
Tamura, S., Suzuki, K., Ito, Y., & Fukawa, A. (2021). Factors related to the resilience and mental health of adult cancer patients: A systematic review. Supportive Care in Cancer, 29(7), 3471–3486. https://doi.org/10.1007/s00520-020-05943-7
van den Beuken-van Everdingen, M.H., Hochstenbach, L.M.J., Joosten, E.A.J., Tjan-Heijnen, V.C.G., & Janssen, D.J.A. (2016). Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. Journal of Pain and Symptom Management, 51(6), 1070–1090.e9. https://doi.org/10.1016/j.jpainsymman.2015.12.340
Venkatesulu, B.P., Chandrasekar, V.T., Girdhar, P., Advani, P., Sharma, A., Elumalai, T., . . . Krishnan, S. (2021). A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectrum, 5(2), pkaa102. https://doi.org/10.1093/jncics/pkaa102
Vermunt, J.K., & Magidson, J. (2002). Latent class cluster analysis. In J.A. Hagenaars & A.L. McCutcheon (Eds.), Applied latent class analysis (pp. 89–106). Cambridge University Press. https://doi.org/10.1017/CBO9780511499531
Weiss, D.S., & Marmar, C.R. (1997). The Impact of Event Scale–Revised. In J.P. Wilson & T.M. Keane (Eds.), Assessing psychological trauma and PTSD (pp. 399–411). Guilford Press.
Weiss, S.J., Franck, L.S., Leutwyler, H., Dawson-Rose, C.S., Wallhagen, M.I., Staveski, S.L., . . . Miaskowski, C.A. (2023). Theory of Symptom Management. In M.J. Smith, P.R. Liehr, & R. Carpenter (Eds.), Middle range theory for nursing (5th ed., pp. 125–141). Springer.
Wright, F., Hammer, M., Paul, S.M., Aouizerat, B.E., Kober, K.M., Conley, Y.P., . . . Miaskowski, C. (2017). Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine, 91, 187–210. https://doi.org/10.1016/j.cyto.2016.12.023
Zhang, H., Zhao, Q., Cao, P., & Ren, G. (2017). Resilience and quality of life: Exploring the mediator role of social support in patients with breast cancer. Medical Science Monitor, 23, 5969–5979. https://doi.org/10.12659/msm.907730
Zhang, Y.-B., Zhong, X.-M., Han, N., Tang, H., Wang, S.-Y., & Lin, W.-X. (2023). Effectiveness of exercise interventions in the management of cancer-related fatigue: A systematic review of systematic reviews. Supportive Care in Cancer, 31(3), 153. https://doi.org/10.1007/s00520-023-07619-4
Zhu, C., Ma, H., He, A., Li, Y., He, C., & Xia, Y. (2022). Exercise in cancer prevention and anticancer therapy: Efficacy, molecular mechanisms and clinical information. Cancer Letters, 544, 215814. https://doi.org/10.1016/j.canlet.2022.215814

