Virtual Reality for Symptom Management in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Quality Improvement Initiative
Objectives: To evaluate the effects of virtual reality (VR) on symptom distress, such as depression, anxiety, and pain, experienced by individuals receiving allogeneic hematopoietic stem cell transplantation.
Sample & Setting: 20 participants aged 19–70 years (median age of 56.5 years) who were hospitalized in an academic setting received as many as two sessions of VR per week for two weeks.
Methods & Variables: Before and after each session, participants completed the revised Edmonton Symptom Assessment Scale (ESAS-r) to evaluate their symptoms. Paired t tests were later conducted.
Results: VR sessions showed significant improvement in 8 of the 10 symptoms addressed in ESAS-r.
Implications for Nursing: VR can improve symptoms in patients following hematopoietic stem cell transplantation in a hospital setting, provide a low-cost intervention to treat symptoms, and support future investigations exploring how VR affects prolonged hospitalizations related to distressing symptoms.
Jump to a section
Hematopoietic stem cell transplantations (HSCTs) in the United States have increased greatly during the past century and are an accepted standard of practice for hematologic disorders (Driscoll et al., 2017; Léger & Nevill, 2004). From 2014 to 2018, there were a total of 108,237 stem cell transplantations in the United States (Human Resources and Services Administration, n.d.). During the first two to three weeks following HSCT, patients can experience significant side effects, such as mouth sores, pain, nausea and vomiting, diarrhea, appetite changes, fatigue, altered mental status, hair loss, and infection (Bevans et al., 2008; Frödin et al., 2015). All patients undergoing HSCT are at risk for these symptoms during their treatment process and recovery; however, patients who receive myeloablative conditioning have a higher risk for severe symptoms (Frödin et al., 2015).
Hematopoietic Stem Cell Transplantation
HSCT aims to prolong life, improve quality of life, and cure disease in patients with hematologic deficiencies (Driscoll et al., 2017). There are three main categories of stem cells transplanted into patients: allogeneic with myeloablative conditioning, allogeneic with nonmyeloablative conditioning, and autologous transplantation (Pei & Huang, 2019). Patients receiving an allogeneic HSCT receive stem cells from a donor who may or may not be related to the patient, whereas autologous HSCTs use stem cells previously collected from the patient prior to transplantation (Marques et al., 2018). Patients who receive HSCT with myeloablative conditioning are subjected to high doses of chemotherapy to depress their bone marrow function prior to transplantation (Marques et al., 2018). Unfortunately, 40%–60% of allogeneic HSCT recipients develop graft-versus-host disease (Frödin et al., 2015; Villarreal et al., 2016). There are numerous physical sequelae from HSCT treatments, and complications can range from mucositis to death.
Symptoms
Psychiatric disorders can be prevalent in patients receiving HSCT. Prieto et al. (2002) assessed psychiatric disorders in 1,062 patients who underwent HSCT, and how these disorders affect the length of hospitalization. The study found the overall prevalence for any adjustment disorder, anxiety, or mood disorder in the HSCT population was 42%. These disorders had a negative impact on comfort and quality of life in patients who received HSCT (Prieto et al., 2002).
Anxiety and depression can have a negative effect on subjective symptom burden, length of hospital stay, and long-term depression in patients undergoing HSCT (El-Jawahri et al., 2015). Kuba et al. (2017) conducted a prospective multicenter study investigating the course of anxiety and depression before and after allogeneic HSCT in 239 patients. Depression rates increased 12%–30% after HSCT, and the long-term effects of depression lasted for almost five years (Kuba et al., 2017). On the other hand, anxiety rates leveled off at three months post-transplantation when compared to the generalized public (Kuba et al., 2017).
Other prospective studies have also found the risk for depression is highest during hospitalization shortly after transplantation (Kuba et al., 2017; Marques et al., 2018; Seo et al., 2019). Seo et al. (2019) conducted a prospective longitudinal study evaluating the psychological symptoms and physical distress experienced by patients after receiving HSCT. Anxiety and depression scores peaked on the seventh day following transplantation, with anxiety rates of 28% and depression rates of 37%. Symptoms, such as nausea, shortness of breath, lack of appetite, and pain, were also associated with increased anxiety (Seo et al., 2019).
An appropriate treatment plan should address symptoms during the recovery phase of HSCT for patients to cope with the aftermath of transplantation and have a better quality of life. Psychiatric symptoms in patients undergoing HSCT are commonly treated with pharmacotherapy, such as antidepressants, anxiolytics, and antipsychotics (Amonoo et al., 2019; Bubalo, 2018). However, there are risks when starting new medications, including adverse effect profiles, drug–drug interactions, and medical comorbidities (Bubalo, 2018). Commonly used antidepressants are known to increase the risk of bleeding in patients undergoing HSCT because of the effects on platelet function (Amonoo et al., 2019). Increased risk for neutropenia, thrombocytopenia, and agranulocytosis are associated with several antipsychotic medications (Amonoo et al., 2019; Nakamura et al., 2019). Patients undergoing HSCT may be unable to swallow oral medications if they have severe chemotherapy-induced mucositis, nausea, or vomiting. Most importantly, centrally acting medications, such as opioids, antidepressants, and anxiolytics, can increase the risk profile for delirium in patients who received HSCT (Nakamura et al., 2019). Delirium after HSCT has been shown to lead to poorer neurocognitive functioning, higher levels of fatigue and distress, and a greater mortality rate (Nakamura et al., 2019).
Cognitive behavioral therapy (CBT), massage therapy, and music therapy are alternatives to medications for anxiety and depression. CBT is a behavioral intervention that entails psychoeducation on effective coping skills to reduce the stressors associated with HSCT treatment and recovery (Amonoo et al., 2019). Although CBT, music, and massage therapy are used in clinical practice, data investigating the efficacy of these interventions in the HSCT population are limited (Bubalo, 2018). Multiple resources and staff would be required to provide CBT, music, or massage therapy, and there could be a financial cost to implement these programs. The prevalence and impact of psychiatric disorders in the HSCT population are well described in the literature; however, there is limited evidence on which interventions provide the most benefit in managing symptoms. Virtual reality (VR) has the potential to combat symptoms like pain, depression, and anxiety in patients recovering from HSCT, while also eliminating the risk factors that are associated with pharmacotherapy.
Virtual Reality
VR has been studied in select populations as an intervention to reduce pain, anxiety, and other symptoms associated with procedures or treatments (Ahmad et al., 2020; Ioannou et al., 2020). VR targets multiple sensory modalities, including auditory, visual, or haptic experiences by using computer-generated scenarios, with which individuals can interact. Headsets or goggles are used to facilitate a perception of reality, which stimulate the senses of the viewer. The entertaining and immersive effects of VR can be helpful in redirecting a patient’s attention from distressing experiences, and reduce pain, anxiety, discomfort, or other symptoms (Dascal et al., 2017; Ioannou et al., 2020). It is vital to study VR in specialized populations, such as HSCT, to determine its efficiency as a coping strategy for symptoms.
VR can cause some side effects, such as perceptuomotor after-effects and simulation sickness, also known as cybersickness (Baniasadi et al., 2020; Kim et al., 2018). Individuals with cybersickness usually exhibit signs of nausea, vomiting, eye fatigue, dizziness, and ataxia after 20 minutes of exposure, which could lead to lack of adherence to VR. Eye strain and headaches have also been reported in individuals who have prolonged exposure with VR systems (Baniasadi et al., 2020). On the other hand, VR sessions can offer a pleasant distraction and block out stimuli, like pain or anxiety, which can improve patients’ quality of life (Ahmad et al., 2020).
Theoretical Model
Kolcaba’s Comfort Theory was used as the theoretical foundation for this project. The theory addresses a patient’s comfort in the context of the four holistic experiences (environment, psychospiritual, physical, and sociocultural) and can be divided into the following three parts: healthcare needs, health-seeking behavior, and institutional integrity (Kolcaba, 2013). Healthcare needs, also known as comfort needs, arise when stressful situations occur and can affect a patient’s psychospiritual, social, physical, and/or environmental experience. Patients who can attain enhanced comfort will improve their health-seeking behavior. Internal behaviors (e.g., cellular level) and external behaviors (e.g., functional capacity, self-care ability, participation in health programs) are key components of health-seeking behaviors. Institutional integrity is defined as the quality of care provided by medical teams, which can be measured according to length of hospitalization, costs of care, and patient satisfaction (Kolcaba, 2013). The comfort theory assumes all individuals respond to complex stimuli with physical, psychospiritual, sociocultural, and environmental reactions. The whole human response is greater than separate, smaller responses to stimuli. In addition, all individuals want to be comfortable and will seek comfort whenever possible (Kolcaba, 2013). The theory suggests that patients will engage in positive health-seeking behaviors when they are more comfortable.
Kolcaba’s Comfort Theory was applied to the VR intervention, which targeted symptoms exhibited in the recovery phase of HSCT. VR was also used to address a patient’s full comfort, which includes improved healthcare needs and health-seeking behaviors, such as increased appetite and decreased use of extra resources to target symptoms.
Quality Improvement Model
The Model for Understanding Success in Quality (MUSIQ) was used as a framework to guide the application of the quality improvement project. MUSIQ provides 25 contextual factors influencing quality improvement success, and classifies these factors based on the type of healthcare institution involved (Kaplan et al., 2011). Possible relationships between the contextual factors and success of the project are also identified by MUSIQ. Quality improvement leadership, staff motivation and capability, and the institution’s culture are some of the microsystem factors influencing a project’s success. In addition, the effectiveness of a project is dependent on multiple components, such as the team’s structure, history of the team working together, expertise of the team, and behavior of all team members (Kaplan et al., 2011). MUSIQ has been used in many quality improvement models because of its attention to these contextual factors, while also recognizing the unique relationships among the different factors in a complex healthcare system (Kaplan et al., 2013; Reed et al., 2018). In the academic setting for this quality improvement project, MUSIQ fostered a problem-solving process to think through the identified problem (i.e., distressing symptoms in patients undergoing HSCT), reduce the gap in clinician knowledge, identify root causes preventing the team from meeting targets, and successful ly create plans of action (e.g., application of VR).
Purpose
The aim of this quality improvement project was to determine if VR can diminish symptoms, such as anxiety and pain, in patients undergoing hospitalization for induction chemotherapy and allogeneic HSCT. In the project setting, distress and symptom burden affecting patients undergoing HSCT were identified by nursing staff during daily interprofessional team rounds and interactions with the palliative care team. High doses of opioids and other symptom-directed medications were noted in many patients undergoing HSCT. Nursing staff independently reached out to palliative care consultation teams to request assistance in treating symptom distress in patients undergoing HSCT. The project was created to evaluate the potential use of VR, a noninvasive intervention, in patients undergoing HSCT given the high rate of distressing symptoms seen in this population. VR was identified as a cognitive distraction tool, which could diminish distressing symptoms in patients undergoing HSCT.
Methods
A pre- and post-test quasi-experimental design was used to examine VR as a possible tool to decrease symptoms in patients undergoing HSCT. Participants were scheduled to receive the VR intervention during their initial hospitalization for allogeneic HSCT. The within-group design was used to control for gender, age, medications (e.g., antiemetics, antidepressants, opioids), and cancer diagnosis.
Setting
The quality improvement project took place in an inpatient setting at an academic healthcare institution, Stanford Health Care, in Stanford, CA. More than 300 transplantations are performed annually at the academic center, and most patients are non-Hispanic, White men. The current process for individuals exhibiting significant symptoms following HSCT includes evaluation and treatment by specialized teams, such as palliative care or pain teams, and administration of symptom-directed medications. Participants were hospitalized on the blood and marrow transplantation unit, which holds as many as 40 patients. All participants were hospitalized for high-dose chemotherapy for HSCT and were isolated in a private room from other patients. To prevent the spread of infection, the infection control committee approved VR headsets to be used in the HSCT population if they were disinfected with a hospital-approved antiseptic before and after patient use. HSCT staff (nurses and physicians) were educated on the quality improvement project through staff meetings and fliers. The project was conducted by the quality improvement project committee that included two advanced practice nurses, one physician, and one researcher.
Sample
Participants were recruited from an academic healthcare institution. The project was reviewed by Stanford Health Care’s Human Research Protection Program, which determined that the project did not meet the guidelines for human subjects research. The project was approved to proceed as a quality improvement initiative and was not required to undergo further review by the institutional review board. Enrollment occurred from September 2020 to March 2021. Based on the historical admission rate of patients in a two-month period, the committee aimed to enroll 20 patients. A power analysis was not conducted or required because this was a quality improvement project. Inclusion criteria were as follows: patients admitted for induction for HSCT, planned hospitalization scheduled for at least two weeks, aged 18 years or older, able to read and write in English, an absence of metastatic or primary disease involving the brain, and no history of seizures, delirium, or motion sickness. Exclusion criteria included any patients who tested positive for COVID-19 or were waiting to be tested. There was no cost to the patients to enroll in the quality improvement project.
Methodologic Approach
Medical oncologists and HSCT nurses identified potential participants on initial admittance to the hospital for transplantation. Medical oncologists and HSCT nurses received a 30-minute introduction on the quality initiative project, VR, and how to refer patients to the project. Investigators involved with the application of VR were given a two-hour introduction on how to apply the VR, clean the googles, and document the results of the intervention. All patients who were admitted for allogeneic HSCT and met criteria were offered the VR intervention by investigators for the quality improvement project. Every participant was administered a survey by a trained investigator immediately before and within 10 minutes after each VR session. The survey was not administrated outside of the VR session or at discharge. Participants were offered VR up to twice per week for at least two weeks. Each session was limited to 20 minutes because of concerns about adverse effects, such has cybersickness (Baniasadi et al., 2020). The investigators assisted each participant with placing the headset on their head, recording the time and date of the VR intervention, adhering to 20 minutes per intervention guidelines, and administering the survey before and after the session. The project manager collected all data from researchers to file into the JMPS software for analysis.
A commercially available VR headset (Oculus) was used. Participants chose from a variety of VR videos from the Samsung and YouTube collections. These videos were categorized as travel, meditation, games, entertainment, and sports. During the 20-minute VR intervention, participants were seated in their hospital bed or a chair. Antiemetics, antidepressants, and pain medications prescribed to participants were recorded.
Data Collection
The 10-item revised Edmonton Symptom Assessment Scale (ESAS-r) was used to evaluate symptom distress in patients undergoing HSCT (Richardson & Jones, 2009). The ESAS-r is a revised version of a similar scale survey that was created as a clinical tool to assess symptom severity as part of a clinical assessment (Bruera et al., 1991). The ESAS-r specifies the current time frame for symptoms, has improved definitions for symptoms, and adds “other symptoms” as a write-in patient-specific symptom. Patients rate 10 symptoms (shortness of breath, nausea, pain, depression, fatigue, drowsiness, anxiety, appetite, quality of life, and well-being) on a scale ranging from 0 (no symptom) to 10 (worst possible symptom). For well-being and appetite, a score of 0 indicates best symptom. Quality of life was added as the tenth symptom, which was found valid and reliable in the oncology population (Chang et al., 2000).
The ESAS-r is a brief survey that requires minimal effort for participants and investigators alike, and is easy to complete in a short period of time (Rees et al., 1998; Richardson & Jones, 2009). It is a self-rated scale that has been validated in patients with cancer and those receiving palliative care (Bruera et al., 1991; D’Ambruoso et al., 2016; Rees et al., 1998). The gold standard for symptom assessment is the patient’s opinion of their symptoms (Rees et al., 1998).
Clinical and demographic characteristics of participants, including ESAS-r scores, cancer diagnosis (ICD-10 codes), sex (male, female, other), race, age (date of birth), prescribed medications, and the patient’s selected VR video, were collected by the project manager after reviewing each participant’s medical chart (see Table 1). Vital signs and ESAS-r scores were documented by the investigator assisting the participant with VR. Data were not collected on length of stay or changes in symptom-directed medications following VR. Data were managed in a Microsoft Excel spreadsheet. Each patient was given a unique number to use across datasets. 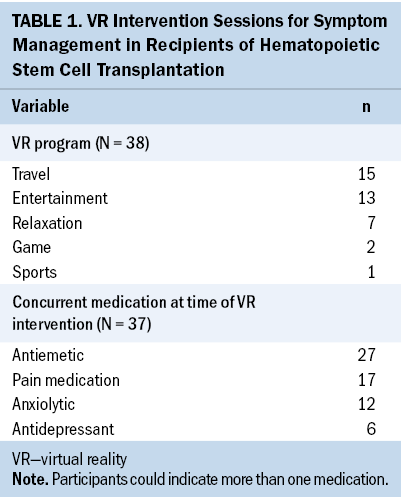
Analysis
The VR intervention took place during a seven-month period. All variables were assessed using descriptive statistics. Means and standard deviations were calculated for continuous variables. Categorical variables were displayed as frequencies or percentages. Paired t tests were used to compare mean ESAS-r scores pre- and postintervention for each separate encounter. JMPS software was used to format and analyze the data. In all analyses, statistical significance was set at p < 0.05.
Results
The median age of participants was 56.5 years, and most identified as male (n = 15) and non-Hispanic White (n = 14). Additional demographic variables are displayed in Table 2. Most participants chose videos depicting entertainment and travel. Thirty-eight VR interventions were recorded from the 20 participants. Only one participant ended their VR session early because of nausea provoked by watching a VR video. 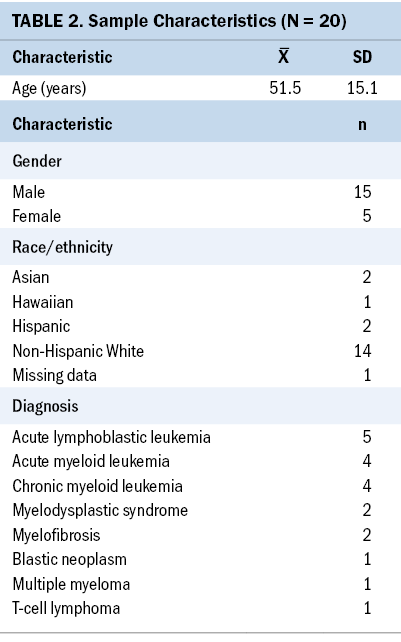
There were significant improvements (p < 0.05) in 8 of 10 symptoms addressed by ESAS-r (depression, tiredness, anxiety, drowsiness, lack of appetite, pain, quality of life, and well-being). Lack of appetite (mean difference score of 1.8) and anxiety (mean difference score of 1.2) had the most profound improvement compared to the other six symptoms (p < 0.0001). Nausea and shortness of breath did not show any statistically significant improvement; however, overall nausea scores appeared to decrease from a score of 1.9 to 1.5, with a mean difference of 0.4. Although reduction in nausea scores were not shown to be statistically significant, it did highlight that most participants did not get nauseated when using the VR headset. Total ESAS-r scores (scores of the 10 items combined) were also evaluated, and statistically significant improvements were found (p < 0.0001; mean difference score of 10) (see Table 3). 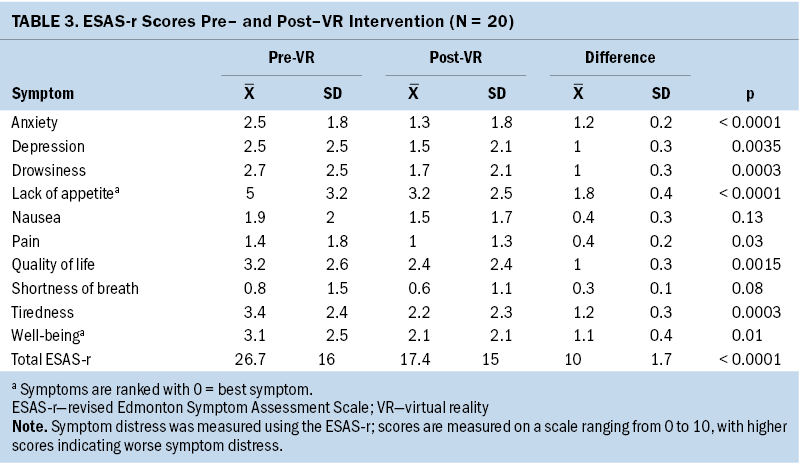
Discussion
In this quality improvement project examining the impact of VR for patients hospitalized for HSCT, the VR intervention was feasible and successful in reducing a variety of symptoms. VR was used in this project to address these symptoms and produced similar results compared to previous VR studies evaluating symptoms in other populations (Ahmad et al., 2020; Chirico et al., 2015). Statistically significant results were seen in eight of the symptoms evaluated by the ESAS-r, and all 10 symptoms improved by some degree. It is important to emphasize the significant improvement seen in appetite during this project. Lack of appetite has been shown as one of the most common symptoms seen in patients following initial transplantation and can remain present for as long as six months (Marques et al., 2018). It is unclear why appetite showed significant improvement; however, many participants viewed travel and entertainment videos, which could have included images of food that sparked their appetites. Schüssler et al. (2012) found that viewing images of food could cause an increase in ghrelin, a hormone that causes hunger.
Overall, ESAS-r scores showed substantial improvement in reduction of symptoms following the VR intervention (from a mean total score of 26.7 preintervention to 17.4 postintervention). Elevated psychological symptoms, such as depression and anxiety, and complications from graft-versus-host disease (pain, nausea, lack of appetite) have been identified as risk factors for prolonged hospitalization and post-transplantation mortality (Godara et al., 2021; Solh, 2020). Only one participant experienced adverse side effects, consistent with previous VR studies showing VR as a well-tolerated intervention. In addition, a few participants shared that VR was a welcomed escape during the COVID-19 pandemic. Reducing overall distressing symptoms could translate to reduced hospital costs and length of stay; however, this quality improvement project did not investigate the cost–benefit ratio in relation to length of stay or reduction in overall prescribed medications.
The cost of VR was reasonable with Oculus headsets, ranging from a few hundred dollars to $1,000 for high-end VR headsets (Robertson, n.d.). The low cost of VR and the reduction in symptom distress supports the use of VR as an effective, cost-efficient intervention to improve quality of life for patients receiving HSCTs. The improvements seen after the use of VR positively influenced administrators and staff of the blood and marrow transplantation unit. The staff requested to continue VR for patients undergoing HSCT following the completion of this project. The identification of key leadership and culture factors highlighted in the MUSIQ model helped facilitate adoption of VR in the blood and marrow transplantation unit. Future meetings with the leadership team and staff are planned to help solidify next steps in implementing VR as a possible standard of care for patients interested in treating their symptoms with VR. Staff members from both the blood and marrow transplantation unit and palliative care team have already been trained on VR and are prepared to provide VR when an action plan is completed.
Limitations
There are several limitations to this project. This project was conducted during the COVID-19 pandemic, which created recruitment difficulties. Some patients who met criteria for enrollment declined because of concerns that the COVID-19 virus could be spread by the VR headset, despite reassurance from infection control that the headset is thoroughly disinfected between participants. Scheduled hospital admissions for HSCTs were also significantly reduced, causing the project to be delayed until hospital enrollment increased. The project only investigated transient effects by evaluating symptoms shortly after the VR intervention. It is unknown if the observed effect of VR intervention is short-lived or sustainable. Therefore, it would be reasonable to study the sustainability of the effect of VR on periodic interventions throughout the entirety of an individual’s hospitalization.
The project was designed as a quality improvement project for the transplantation unit rather than a formal research study, making results potentially less generalizable. For instance, the project used a single setting with a small number of participants and only examined individuals hospitalized to receive an allogeneic HSCT. In addition, participants identified predominantly as White and male. Future work could extend these results by conducting larger, multisite studies with more diverse participants, employing more rigorous experimental designs (e.g., randomized controlled trials), and examining the effect of VR in patients hospitalized for other conditions.
The project lacked a standardized time frame for VR intervention because participants were able to watch anywhere from a few minutes to 20 minutes of VR videos. Participants could also choose to use the VR intervention as many as four times in a two-week hospitalization period, making it difficult to determine whether patients had enough exposure to the intervention to produce an effect on symptoms. A longer exposure to VR may be needed to invoke a stronger effect on symptoms. Lastly, not all participants completed all four sessions. Five participants completed three to four sessions, whereas 15 completed one to two sessions. There are multiple potential reasons for lack of adherence to VR, including a brief pause in the quality improvement project during the height of the COVID-19 pandemic, early discharge, lack of interest, or interference of other activities when VR was offered.
Implications for Nursing
VR was found to be a feasible distraction intervention to implement in the hospital setting. Most of the project’s participants did not report any symptoms, such as headaches, dizziness, or visual disturbances, with VR. Despite the minimal side effects, staff should monitor VR and administer with caution. Future research should continue to explore how VR can affect symptom distress in hospitalized patients, the hospital’s costs, and length of stay. In addition, it would be relevant to investigate the effects of repeated exposure to VR to determine whether long-term use of VR could have prolonged symptom relief. 
Conclusion
This project found that VR can diminish symptoms, such as anxiety, depression, and pain, in patients undergoing hospitalization for induction chemotherapy and allogeneic HSCT. There were minimal side effects seen with VR, with only one participant indicating significant nausea during a VR session. A few participants indicated videos retrieved from YouTube were blurry, which may have negatively influenced the participants’ VR experience. Using professionally produced videos may improve the VR experience and better influence patients’ symptoms. Other institutions considering implementing a VR program will require a team of clinicians to administer the VR, maintain and clean VR goggles, and conduct documentation. Staff can easily be trained on the application of VR goggles with a brief one- or two-hour introduction course. Although the cost of VR goggles is low, there could be a financial cost to hire staff to run a VR program. The quality improvement project’s results support the theory that watching VR videos can make hospitalizations more tolerable and possibly improve symptoms; however, clinicians should not always assume symptoms will improve.
About the Author(s)
Colleen Vega, DNP, CNS, ACHPN®, is a clinical nurse specialist at Stanford Health Care in San Jose, CA; Robin L. Whitney, PhD, RN, is an assistant professor in the School of Nursing at San Jose State University in Fresno, CA; and Josef Hannah, DO, is a clinical assistant professor and physician in the School of Medicine, and Grant Matthew Smith, MD, is a physician in the School of Medicine, both at Stanford University in Stanford, CA. No financial relationships to disclose. Vega, Whitney, and Hannah contributed to the conceptualization and design. Vega and Hannah completed the data collection. Vega and Smith provided statistical support and analysis. All authors contributed to the manuscript preparation. Vega can be reached at covega@standfordhealthcare.org, with copy to ONFEditor@ons.org. (Submitted May 2021. Accepted October 22, 2021.)

