Association Between Patient-Reported Symptoms of Dysphagia and Psychological Distress in Head and Neck Cancer Survivors
Objectives: To describe the prevalence of and the association between patient-reported dysphagia and psychological distress (anxiety and depression) in head and neck cancer (HNC) survivors.
Sample & Setting: 228 HNC survivors seen at an interprofessional survivorship clinic in Pittsburgh, PA, between October 2018 and January 2020.
Methods & Variables: Dysphagia was evaluated using the Eating Assessment Tool. Anxiety and depression were measured using the Generalized Anxiety Disorder–7 and Patient Health Questionnaire–8, respectively. Descriptive statistics and multiple linear regression were performed.
Results: 70% (n = 159) of survivors reported problems with swallowing safely and efficiently. Twenty-seven survivors reported symptoms of major depression, 34 reported mild symptoms of anxiety, and 19 reported moderate to severe symptoms of anxiety and depression. After controlling for treatment modality, age, and stage, dysphagia was associated with increased symptoms of anxiety and depression.
Implications for Nursing: Oncology nurses can inform their daily practice by implementing regular assessments for anxiety and depression in HNC survivors reporting symptoms of dysphagia.
Jump to a section
Head and neck cancer (HNC) generally originates in the squamous cells of the upper aerodigestive tract. The most common sites include the oral cavity, pharynx, and larynx (National Cancer Institute [NCI], 2021). HNC diagnoses comprise about 4% of total cancer diagnoses in the United States, and more than 70% of those diagnosed are men (NCI Surveillance, Epidemiology, and End Results Program, 2021; Siegel et al., 2020). Treatment for early-stage HNC commonly includes either surgery or radiation therapy. However, about 60% of patients are diagnosed with advanced disease, which is typically categorized by a larger primary tumor volume, metastases to the regional lymph nodes, or a combination of both (Chow, 2020). The treatment method for advanced stage carcinoma is often multimodal, including a combination of radiation therapy, chemotherapy, and/or surgery (Marur & Forastiere, 2016).
HNC and its treatment can contribute to high symptom burden, which can interfere with patients’ daily activities (Gunn et al., 2013; Hanna et al., 2015). About one-third of patients with HNC present to treatment with high symptom burden, with the most severe symptoms reported being pain, fatigue, distress, and insomnia (Hanna et al., 2015). Patients undergoing concurrent chemotherapy and radiation therapy are more likely to experience a higher symptom burden than patients undergoing radiation therapy alone (Rosenthal et al., 2014). Because of the routine use of multimodal therapy, substantial side effects are common. The most prevalent toxicities include fibrosis of the head and neck region, xerostomia, dysgeusia, psychological distress, pain, and dysphagia (Astrup et al., 2015; Haisfield-Wolfe et al., 2009; Nilsen et al., 2019; Villa & Sonis, 2016).
Dysphagia, or swallowing dysfunction, is a medical condition in which individuals have difficulty safely or efficiently advancing solids or liquids from the oral cavity to the stomach (Rommel & Hamdy, 2015). Commonly described during and after treatment, dysphagia is one of the most devastating side effects related to HNC treatment, and it is associated with increased morbidity and mortality (Rommel & Hamdy, 2015). Swallowing dysfunction can cause coughing or choking during meals, may lead to aspiration pneumonia, and could necessitate alternate feeding methods (e.g., feeding tube). These consequences may contribute to psychosocial distress, such as embarrassment, isolation, and symptoms of psychological distress, including anxiety, depression, and decreased quality of life (El-Deiry et al., 2009; Rommel & Hamdy, 2015). According to a Surveillance, Epidemiology, and End Results–Medicare analysis that explored the two-year prevalence of dysphagia among 16,194 patients with HNC, the incidence of dysphagia was as high as 45.3% (Hutcheson et al., 2019). Prevalence has increased by about 11.7% during the previous decade (Hutcheson et al., 2019). Considering these data, dysphagia presents a very large symptom burden among HNC survivors.
Psychological distress is defined by symptoms of generalized anxiety and/or major depression (Hamer et al., 2009). Patients with HNC, as well as HNC survivors, experience one of the highest rates of depression among all people with cancer (Lydiatt et al., 2009), and symptoms of anxiety are very common long-term effects (Cohen et al., 2016). It is estimated that the incidence of depression among individuals with HNC ranges from 10% to 50%, far surpassing that of individuals with other cancer types (Chen et al., 2013; Lydiatt et al., 2009; Rieke et al., 2017; Wu et al., 2016). Although a specific incidence of anxiety among HNC survivors is difficult to determine, one systematic review found that the prevalence of anxiety among all individuals with cancer is about 17.9% (Mitchell et al., 2013). Therefore, depression and anxiety also lead to a high symptom burden in the HNC population.
Despite knowing the effects that dysphagia, anxiety, and depression have on HNC survivors, little research has been conducted regarding the association between patient-reported swallowing dysfunction (dysphagia) and psychological distress in HNC survivors. This is the first cross-sectional study to evaluate the relationship between patient-reported dysphagia and symptoms of anxiety and depression among HNC survivors. By expanding knowledge of this association, oncology nursing practice can be improved by implementing additional screening for depression and anxiety in survivors reporting swallowing dysfunction. The purpose of this study was to (a) describe the prevalence of patient-reported symptoms of dysphagia and psychological distress (i.e., symptoms of anxiety and depression) and (b) explore the association between patient-reported dysphagia and psychological distress in HNC survivors.
Methods
A cross-sectional analysis of adult (aged 18 years or older) HNC survivors was conducted between October 2018 and January 2020. As a part of their clinical evaluation in the outpatient, interprofessional University of Pittsburgh Medical Center Head and Neck Cancer Survivorship Clinic in Pennsylvania, each survivor completed patient-reported outcomes questionnaires regarding swallowing dysfunction and symptoms of anxiety and depression. All participants provided informed consent to be included in various research studies regarding HNC survivorship through the interprofessional clinic. To be included in this analysis, survivors had to be at least one year postcompletion of primary treatment for squamous cell carcinoma of the oral cavity, oropharynx, or larynx/hypopharynx. Survivors were excluded from the study if they had a history of recurrence, second primary cancer, or distant metastases. Figure 1 illustrates the CONSORT (Consolidated Standards of Reporting Trials) flow diagram for this study. 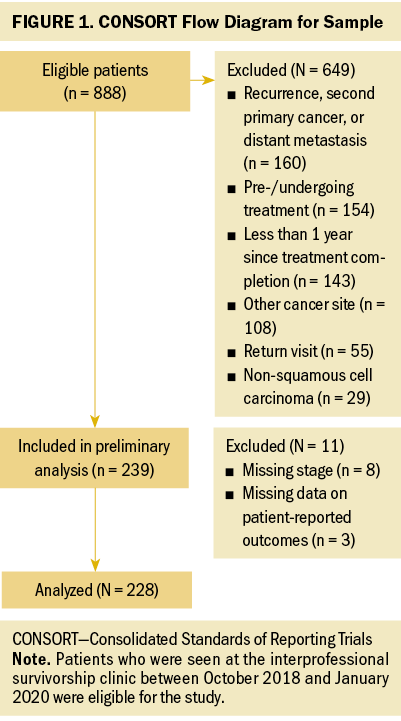
Demographics and Clinical Characteristics
Demographic information was obtained from medical record review and included age, gender, marital status, and race; clinical characteristics included tumor site (oral cavity, oropharynx, larynx/hypopharynx), stage at time of diagnosis, treatment modality (surgery alone, nonsurgical [radiation therapy or concurrent chemotherapy and radiation therapy], surgery and adjuvant treatment [radiation therapy or concurrent chemotherapy and radiation therapy]), and time since completion of treatment.
Dysphagia
Patient-reported symptoms of dysphagia were measured using the 10-item Eating Assessment Tool (EAT-10). Each question has five response options ranging from 0 (no problem) to 4 (severe problem). The questions address swallowing-related weight loss, going out for meals, difficulty with consistencies (i.e., liquids, solids, and pills), pain, the pleasure of eating, food sticking, and coughing. EAT-10 scores are calculated by summing scores on each of the 10 questions, with a total minimum score of 0 and a maximum score of 40. A score of 3 or higher is indicative of disordered swallowing (Belafsky et al., 2008). The EAT-10 questionnaire has established reliability and validity (Belafsky et al., 2008), with substantial use as a patient-reported outcome measure in individuals with HNC (Arrese et al., 2017; Cates et al., 2021; Florie et al., 2021; Likhterov et al., 2018).
Anxiety and Depression
Symptoms of anxiety were measured using the Generalized Anxiety Disorder–7 (GAD-7), which assesses symptoms of anxiety during the past two weeks. The GAD-7 consists of seven questions, each with four response options ranging from 0 to 3 (0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly every day). Question domains include feelings of nervousness or anxiousness, uncontrollable worrying, worrying about different things, trouble relaxing, restlessness, being easily annoyed or irritable, and feeling afraid of something horrible happening. Total scores are calculated by summing all responses, with total scores ranging from 0 to 21. Scoring is interpreted as follows: a score of 0–4 indicates minimal anxiety, a score of 5–9 indicates mild anxiety, a score of 10–14 indicates moderate anxiety, and a score of 15–21 indicates severe anxiety (Anxiety and Depression Association of America, 2020). The GAD-7 had been noted to be psychometrically sound (Plummer et al., 2016) and has been used as a screening measure for generalized anxiety in cancer populations (Esser et al., 2018; Naser et al., 2021; Sarkar et al., 2015).
The Patient Health Questionnaire–8 (PHQ-8) was used to measure symptoms of depression during the past two weeks among survivors. The PHQ-8 consists of eight questions, each scored on a scale ranging from 0 to 3 (0 = not at all, 1 = several days, 2 = more than half the days, 3 = nearly every day). Total scores range from 0 to 24. A total score of 5–9 represents mild depressive symptoms, a score of 10–14 represents moderate symptoms, and a score of 15–24 represents severe depressive symptoms (Shin et al., 2019). The PHQ-8 covers topics, such as pleasure in doing things, feeling down or hopeless, irregular sleeping patterns, feeling tired, irregular eating patterns, feeling bad about oneself, trouble concentrating, and moving too slow or too fast. Previous research supports the usefulness of the PHQ-8 as a screening measure for depression (Kroenke et al., 2009; Smith et al., 2010), with good sensitivity and specificity (Wu et al., 2020).
Statistical Analyses
All statistical analyses were performed using RStudio, version 1.3.1056. In the descriptive analysis, means and standard deviations were computed for continuous variables, and frequencies and percentage were calculated for categorical variables. Regarding the two outcomes of interest (GAD-7 and PHQ-8 scores), overall means, standard deviations, frequencies, and percentages are reported. Scores were categorized into three groups based on the following criteria:
• For GAD-7 scores, scores of 0–4 (no anxiety), scores of 5–9 (mild anxiety), and scores of 10–21 (moderate to severe anxiety)
• For PHQ-8 scores, scores of 0–4 (no depression), scores of 5–9 (mild depression), and scores of 10–24 (moderate to severe depression)
In the univariate analysis and the linear regression analysis, GAD-7 and PHQ-8 scores were treated as continuous variables.
Differences in GAD-7 scores by categorical demographic and clinical characteristics were examined using the Kruskal–Wallis test, and similar tests were performed for PHQ-8 scores. Linear regression analysis was performed to investigate the association between GAD-7 and EAT-10 scores while controlling for treatment modality, age, and stage. A similar linear regression model was fitted to examine the association between PHQ-8 and EAT-10 scores. Sandwich variance estimator was used to estimate the standard errors for both models.
Results
Sample Characteristics
The sample consisted of 228 HNC survivors. Most survivors were men (n = 180, 79%), White (n = 216, 95%), and married (n = 157, 69%). The mean age of survivors was 64.95 years (SD = 10.2 years). The most common tumor site was the oropharynx (n = 127, 56%), followed by the larynx/hypopharynx (n = 52, 23%), and oral cavity (n = 49, 22%). Most survivors (n = 183, 80%) had advanced stage (stage III–IV) cancer at the time of diagnosis. The majority of survivors received a combination of surgery and adjuvant treatment (n = 97, 43%), followed by nonsurgical treatment (n = 88, 39%). Few survivors (n = 43, 19%) were treated with surgery alone. The average time since treatment completion was 6.28 years (SD = 5.92 years). See Table 1 for the demographic and clinical characteristics. 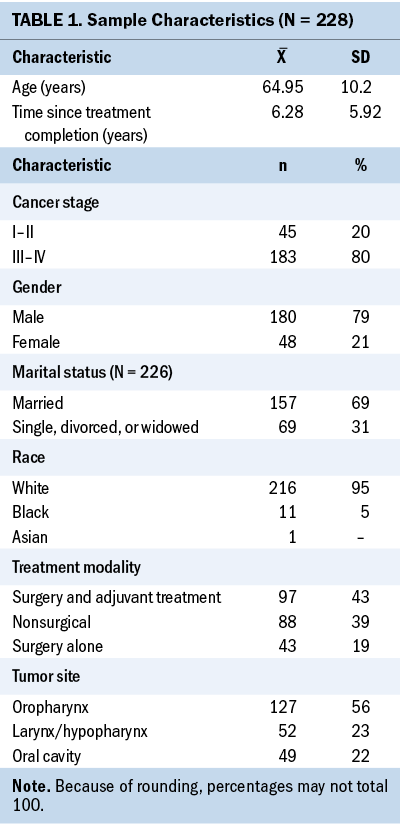
Of the 228 HNC survivors in the sample, 159 (70%) scored a 3 or higher on the EAT-10, indicating swallowing dysfunction. The mean reported score was 9.81 (SD = 10.06), with a range of 0–38. The overall mean GAD-7 score was 2.81 (SD = 4.34), with scores ranging from 0 to 21. Seventy-seven percent (n = 175) of survivors scored 0–4 points on the GAD-7, indicating no symptoms of anxiety; 15% (n = 34) scored 5–9 points, indicating mild symptoms; and 8% (n = 19) scored 10–21 points, indicating moderate to severe symptoms. No correlation was found between GAD-7 scores and race, treatment modality, or tumor site. However, there was a significant correlation between stage and GAD-7 scores (p = 0.002), indicating that individuals with advanced stage HNC are more likely to have greater symptoms of anxiety. Similarly, there was a correlation between GAD-7 scores and marital status (p = 0.029), indicating that unmarried individuals are more likely to report symptoms of anxiety. In addition, a correlation was found between GAD-7 scores and sex (p = 0.029), which indicated that men are more likely than women to report symptoms of anxiety (see Table 2). 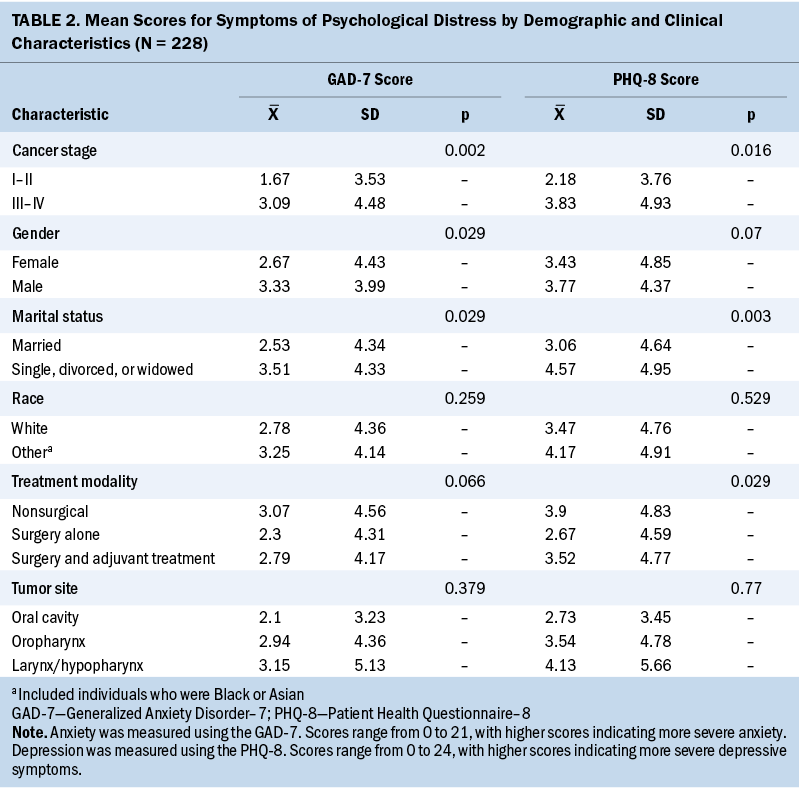
The overall mean PHQ-8 score was 3.5 (SD = 4.76), and total scores ranged from 0 to 21. Seventy-two percent (n = 163) of survivors scored 0–4 points, indicating no symptoms of depression. Comparatively, 17% (n = 38) scored 5–9 points, indicating mild symptoms, and 12% (n = 27) scored 10–21 points, indicating moderate to severe symptoms of depression. No correlation was found between PHQ-8 scores and sex, race, or tumor site. However, there were significant correlations between PHQ-8 scores and marital status (p = 0.003), as well as stage (p = 0.016) and treatment modality (p = 0.029), which suggests that survivors who are unmarried or have more advanced cancer are more likely to report more symptoms of depression. Survivors who were treated with surgery alone were more likely to report fewer symptoms of depression when compared to survivors treated nonsurgically or with a combination of surgery and adjuvant care.
Dysphagia and Psychological Distress
After controlling for treatment modality, age, and stage, more severe symptoms of anxiety and depression were strongly associated with higher EAT-10 scores. The mean EAT-10 score for survivors not reporting symptoms of anxiety was 7.23 (SD = 7.94). Survivors who reported mild symptoms of anxiety (GAD-7 score ranging from 5 to 9) had a mean EAT-10 score of 15.21 (SD = 10.96). Lastly, survivors reporting moderate to severe symptoms of anxiety (GAD-7 score ranging from 10 to 21) had a mean EAT-10 score of 23.95 (SD = 10.75). GAD-7 scores were expected to increase by 0.25 points for every one-point increase in EAT-10 scores after controlling for treatment modality, age, and stage (p < 0.001) (see Table 3). 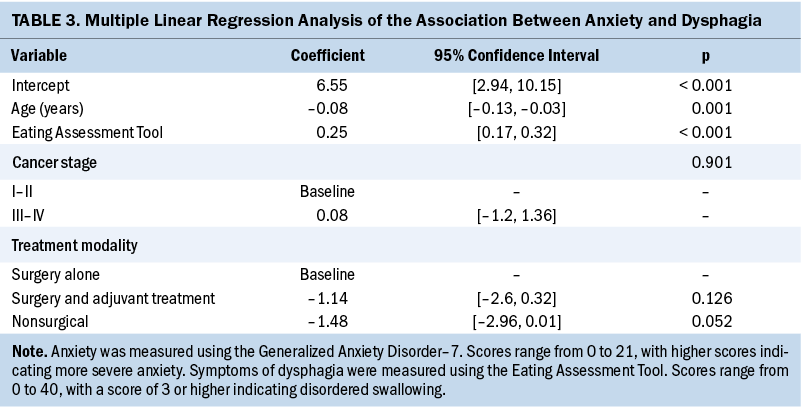
Survivors reporting mild symptoms of depression (PHQ-8 score ranging from 5 to 9) had a mean EAT-10 score of 13.05 (SD = 9.32). Similarly, survivors reporting moderate to severe symptoms of depression (PHQ-8 score ranging from 10 to 24) had a mean EAT-10 score of 23.78 (SD = 10.81). In comparison, survivors who did not report symptoms of depression had EAT-10 scores that were significantly lower than those reporting symptoms (mean = 6.74, SD = 7.67). After controlling for treatment modality, age, and stage, PHQ-8 scores were expected to increase by 0.3 points for every one-point increase in EAT-10 scores (p < 0.001) (see Table 4). 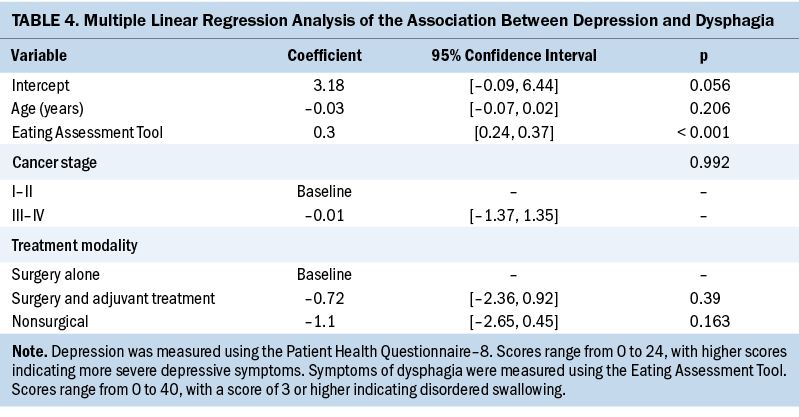
Discussion
Dysphagia is a common side effect of HNC and its treatment (Kronenberger & Meyers, 1994; Murphy & Gilbert, 2009). Many studies have shown that patients with HNC experience a particularly high rate of dysphagia (23%–100%) compared to other patients in the oncology and general populations (1%–13%) (García-Peris et al., 2007; Roden & Altman, 2013). In the current study, 70% (n = 159) of HNC survivors reported symptoms of dysphagia using the EAT-10. Comparatively, the prevalence of dysphagia in the general population ranges from 1.7% to 11.3% depending on varying demographic information (Roden & Altman, 2013). In one study of outcomes related to dysphagia in 104 patients with HNC, swallowing dysfunction was associated with decreased quality of life (Nguyen et al., 2005). In the same study, it was reported that patients with no or mild dysphagia experienced significantly less anxiety and depression than those with moderate to severe dysphagia (p = 0.005 and p = 0.0001, respectively), which is consistent with the results of the current study (Nguyen et al., 2005). These results were attained based on anxiety and depression evaluations using the University of Washington Quality of Life Questionnaire (Nguyen et al., 2005). To the authors’ knowledge, the current study is the largest cross-sectional analysis exploring the association between swallowing dysfunction and symptoms of anxiety and depression in HNC survivors.
Similar to patients with HNC and HNC survivors, individuals with Parkinson disease experience dysphagia. In a study of 127 patients with Parkinson disease by Han et al. (2011), it was found that as the severity of dysphagia increased, symptoms of depression became more severe. Another study involving patients with Parkinson disease was also able to associate more severe symptoms of anxiety with increased swallowing dysfunction (Manor et al., 2009).
HNC has been identified as a type of cancer that is highly associated with depression and anxiety (Massie et al., 2010), which led the current authors to hypothesize that dysphagia could be associated with more severe symptoms of anxiety and depression in HNC survivors. In this study, 23% (n = 53) and 29% (n = 65) of survivors reported symptoms of anxiety and depression, respectively. When comparing anxiety and depression levels in survivors who reported symptoms of dysphagia versus those who did not, statistical analysis showed a substantial positive correlation between increased swallowing dysfunction and more severe symptoms of anxiety and depression. In particular, for each one-point increase in EAT-10 scores, GAD-7 (anxiety) scores were expected to increase by 0.25 points, and PHQ-8 (depression) scores were expected to increase by 0.3 points. Therefore, HNC survivors who self-report symptoms of dysphagia are more likely to disclose symptoms of anxiety and depression.
Limitations
This study has some limitations. Because the obtained data were cross-sectional, no causal association between patient-reported symptoms of dysphagia and psychological distress can be inferred. Therefore, future studies would benefit from a longitudinal approach, which would allow for the evaluation of a cause-and-effect relationship, as well as provide information about individuals’ symptoms over time. In addition, the self-report questionnaires for dysphagia, anxiety, and depression are designed to screen for symptoms, not diagnosis conditions. Because of the design of this specific interprofessional HNC survivorship clinic, it could be true that some patients had a more complex symptom and treatment profile than others, which may have introduced bias. Lastly, because the sample was obtained at one specific clinic, the patients were racially homogeneous. Future studies should acquire a racially diverse sample to better explore the influence of race on these variables.
Implications for Nursing
Oncology practitioners can implement early screening and intervention for psychological distress, such as anxiety and depression, for HNC survivors reporting symptoms of dysphagia. According to a study by Naughton and Weaver (2014), the first one to three years after cessation of treatment are the most critical for a cancer survivor’s mental health, so early action is essential. It is known that anxiety and depression are significant symptoms in the HNC community and that patients with HNC report suicide rates that are significantly higher than in the general population (Kam et al., 2015). Based on the results of the current study, HNC survivors who report symptoms of dysphagia are more likely to also report symptoms of anxiety and depression. Therefore, it is vital that oncology nurses inform their daily practice and implement regular monitoring and assessment for signs and symptoms of anxiety, depression, and suicidal ideations in HNC survivors who report these symptoms. Future studies can be conducted regarding which method of intervention is best for addressing mental health in HNC survivors. When treating individuals who are struggling with symptoms of anxiety and depression, oncology nurses should be empowered to provide the patient with the necessary resources to obtain interventions, such as cognitive behavioral therapy, mindfulness therapy, and/or medication, as needed. In addition, basic psychological care should be implemented into the care plan of all HNC survivors who are experiencing dysphagia. Attention must be brought to the psychological complications associated with this common post-treatment side effect. 
Conclusion
This study demonstrated that patient-reported swallowing dysfunction is significantly associated with increased symptoms of anxiety and depression. The results provide the means to execute a system-level adoption of psychological screening in HNC survivors who report symptoms of dysphagia. Doing so would help practitioners to evaluate and care for symptoms of anxiety and depression early for the benefit and well-being of patients. Further prospective research is needed to replicate these results and to better understand the effects of swallowing dysfunction on mental health among HNC survivors.
About the Author(s)
Kaitlyn Eastburn, SN, is a student nurse in the Department of Acute and Tertiary Care in the School of Nursing; Lingyun Lyu, MS, was, at the time of writing, a graduate student researcher in the Department of Biostatistics in the School of Public Health; Christine Harrison, BS, is a clinical research coordinator, and Karley Atchison, MA, is a research coordinator, both in the Department of Otolaryngology in the School of Medicine; Kelly Moore, MA, CCC-SLP, and Sarah Pomfret, MS, CCC-SLP, are speech language pathologists in the Division of Speech–Language Pathology in the Department of Otolaryngology at the University of Pittsburgh Medical Center; Jonas Johnson, MD, is a surgeon, professor, and chairman of the Department of Otolaryngology in the School of Medicine; and Marci Lee Nilsen, PhD, RN, CHPN®, is an assistant professor in the Department of Acute and Tertiary Care in the School of Nursing, all at the University of Pittsburgh in Pennsylvania. No financial relationships to disclose. Eastburn, Johnson, and Nilsen contributed to the conceptualization and design. Eastburn, Harrison, Atchison, Johnson, and Nilsen completed the data collection. Lyu, Johnson, and Nilsen provided statistical support. Eastburn, Lyu, Moore, and Nilsen provided the analysis. Eastburn, Lyu, Harrison, Moore, Pomfret, Johnson, and Nilsen contributed to the manuscript preparation. Nilsen can be reached at mlf981@pitt.edu, with copy to ONFEditor@ons.org. (Submitted February 2021. Accepted July 22, 2021.)

