Determinants of Quality of Life in Individuals With a Dual Diagnosis of Resectable Pancreatic Cancer and Diabetes Mellitus
Objectives: To explore the associations among clinical characteristics, fatigue, diabetes mellitus (DM) self-care activities, and quality of life (QOL) in individuals with resectable pancreatic cancer and DM.
Sample & Setting: 57 individuals with resectable pancreatic cancer and DM from an outpatient pancreatic surgical department in Taiwan were included in the final analysis.
Methods & Variables: A cross-sectional, correlational design was used. QOL, fatigue, and DM self-care were measured by the European Organisation for Research and Treatment of Cancer QOL Questionnaire–Core 30, the Fatigue Symptom Inventory, and the Summary of Diabetes Self-Care Activities.
Results: Participants who had a shorter duration of DM and higher levels of fatigue (including intensity, duration, and interference) reported lower QOL scores. Participants who performed more DM self-care activities and physical activity per week had higher QOL scores. Fatigue, DM self-care activities, and DM duration were significant factors related to QOL.
Implications for Nursing: Shorter DM duration, increased fatigue, and fewer DM self-care activities were determinants of worse QOL in individuals with resectable pancreatic cancer and DM.
Jump to a section
Cancer and diabetes mellitus (DM) are common and serious chronic diseases that affect health-related outcomes worldwide (World Health Organization, 2020, 2021). As compared to other common cancers (e.g., lung, breast, colorectal), individuals with pancreatic cancer have a significantly higher prevalence of DM (68%) (Aggarwal et al., 2013). One of the treatment options for pancreatic cancer is pancreaticoduodenectomy (PD) (Puckett & Garfield, 2020). In patients who have undergone PD for pancreatic or ampullary cancer, about 12%–54% experience DM (Beger et al., 2018; Eom et al., 2013). In patients post–total pancreatectomy for cancer, 100% experience DM (Juel et al., 2021; Maeda & Hanazaki, 2011). The high prevalence of DM among individuals with pancreatic cancer can result in worse patient-reported outcomes and increase the burden on the healthcare system. In addition, with advancements in pancreatic surgery techniques and postoperative health care, individuals with resectable pancreatic malignancies have an increased chance of living longer (Zhang et al., 2016). Understanding the links among patients’ perceived quality of life (QOL), the concurrent burden of pancreatic cancer (e.g., symptoms), and DM (e.g., daily self-care) is essential.
DM self-care includes a range of activities, such as self-monitoring of blood glucose, taking medications, having a healthy diet, and performing regular physical activity to control the levels of blood glucose (Toobert et al., 2000). According to Hershey et al. (2012), individuals with solid tumors and DM who underwent eight weeks of chemotherapy have difficulties in performing DM self-care activities. Persistent and adequate daily self-management of DM to stabilize serum glucose metabolism is essential for individuals with cancer and DM. Adequate DM self-care not only can delay the onset of DM-related complications (American Diabetes Association, 2020), but also can likely play a crucial role in preventing tumor cell resistance to therapy (Duan et al., 2014). In addition, poor glycemic control (i.e., hyperglycemia) may promote an acidic microenvironment, which may be favorable for cancer cell growth and is likely to increase resistance to cancer cell apoptosis (Duan et al., 2014; Hammer et al., 2019). Understanding the effect of DM self-care activities in individuals with pancreatic cancer and DM is essential to improve treatment outcomes and QOL.
As compared to individuals with cancer but without DM, individuals with both cancer and DM reported having more symptoms, including fatigue (Vissers et al., 2016). Fatigue is a persistent symptom that disrupts physical and emotional aspects of well-being (Scott et al., 2011). In individuals who have had pancreatic resection for malignancy, about 82% reported symptoms of fatigue (Cloyd et al., 2017). In people undergoing adjuvant chemotherapy treatment after pancreatic or periampullary cancer surgery, fatigue is ranked the most frequently reported symptom (Gustavell et al., 2020). Similarly, fatigue is highly prevalent (40%–60%) in individuals with DM and is associated with lower QOL (Finsterer & Mahjoub, 2014; Fritschi & Quinn, 2010; Goedendorp et al., 2014) and worse glycemic control outcomes (García et al., 2015; Park et al., 2015). Because individuals with pancreatic cancer and DM have reported fatigue to be disrupting their QOL, it is important to further investigate the relationship between fatigue and QOL in this population.
In previous studies involving individuals with a dual diagnosis of cancer and DM, the focus remains on comparing QOL between individuals with cancer and DM to those with cancer but not DM; in addition, the most studied cancer types include only prostate, colorectal, and breast cancers (Vissers et al., 2016). Studies in people post–pancreatic cancer surgery (Cloyd et al., 2017; Fong et al., 2017) have not explored the effects of DM and glycemic control on QOL. Similarly, studies in individuals with DM (Li et al., 2019; Nielsen et al., 2016) have not assessed the effects of pancreatic cancer, its related treatments, and symptom burden on QOL. Because pancreatic cancer, DM, and their subsequent treatments can have a negative effect on the experience of fatigue, DM self-care activities, and QOL (Bauer et al., 2018; Scollan-Koliopoulos et al., 2013), it is critical to further explore the determinants of QOL in individuals with a dual diagnosis of pancreatic cancer and DM so that nurses can provide effective interventions to improve QOL. The purposes of this study were to explore (a) the correlation among demographic and clinical characteristics, fatigue, DM self-care activities, and QOL, and (b) the determinants of QOL in individuals with a dual diagnosis of resectable pancreatic cancer and DM.
The authors have built a framework to guide this study according to the following study results in the population of individuals with pancreatic cancer and DM. In individuals with pancreatic cancer, older age (Belyaev et al., 2013), lower functional performance status (Velanovich & Wollner, 2011), shorter postoperative time (Heerkens et al., 2016), and higher levels of fatigue (Lewis et al., 2018) are associated with lower QOL. In individuals with DM, older age, female sex, a higher number of comorbidities, more education (Adriaanse et al., 2016; Wan et al., 2016), a higher body mass index (BMI), longer duration of DM (Jing et al., 2018), higher fatigue (Fritschi & Quinn, 2010), fewer DM self-care activities (Adriaanse et al., 2016), and higher glycated hemoglobin (HbA1c) levels (Shim et al., 2012) are associated with lower QOL. The current study framework includes demographic and clinical factors (i.e., age, sex, education, comorbidities, BMI, postoperative time, DM duration, performance status, and HbA1c), fatigue, and DM self-care activities as possible factors related to QOL in individuals with a dual diagnosis of resectable pancreatic cancer and DM.
Methods
Design and Sample
This was a cross-sectional, correlational study using purposive sampling at an outpatient pancreatic surgical department in a medical center in the National Taiwan University Hospital in Taipei. The medical center had about 1,800 medical-surgical beds and about 100 outpatient visits to the pancreatic surgical department per session, which is typically about four hours. Individuals with resectable pancreatic cancer who visited the outpatient surgical department and who also had DM were referred to the DM outpatient department. This was part of a study that compared fatigue and QOL among adults who had undergone total or partial pancreatic surgery for benign or malignant pancreatic tumors (Kuo et al., 2019). The current study was approved by the institutional review board at the study site (identification number: 201611038RIN) and was registered at ClinicalTrials.gov (identification number: NCT02985502). People who (a) had undergone pancreatic surgery, (b) were diagnosed with DM, (c) were aged 20 years or older, (d) could communicate in Chinese or Taiwanese, and (e) agreed to participate in the study and had signed the informed consent were recruited in the study.
Before conducting the data analysis, a power analysis with G*Power, version 3.1, was performed to estimate the required sample size for a regression analysis. With R2 of 0.3 (estimated from previous studies on QOL in the cancer and DM population) (Adriaanse et al., 2016; Beijer et al., 2008), power of 80%, and alpha of 0.05, and entering eight potential factors (i.e., fatigue intensity, fatigue interference, fatigue duration, diet, physical activity, medication, self-monitoring of blood glucose, and foot care) in the model, a total sample of 44 was required to reach adequate power.
The data were collected by a master’s-prepared research assistant who had three years of experience as an RN in an endocrinology ward, and one year of research assistant and interview skills training in the oncology ward and pancreatic surgical outpatient department. All patients with appointments at the outpatient pancreatic surgical department who met the inclusion criteria were approached and invited to participate. The participants completed the questionnaires by responses to the interviewer during face-to-face interviews that took about 15–20 minutes to complete in a vacant room at the outpatient pancreatic surgical department. The collected data were de-identified by assigning unique codes for each participant. The data were stored in a computer locked with a set of secure account numbers and passwords, and documents were stored and locked in a compartment for which only the principal investigator had the keys.
Study recruitment took place from November 2016 to May 2018. Of the 80 people meeting the criteria who were approached, 5 people refused to enter the study (refusal rate = 6.3%), leaving a total of 75 eligible participants. For the purpose of this study, people who had a benign pathology report for pancreatic tumors (n = 18) were excluded. Individuals with exocrine and endocrine pancreatic cancers who underwent surgery were included in this study. Individuals with confirmed intraductal papillary mucinous carcinoma were also included because they had a malignant pathology report and underwent similar surgical treatments as individuals with other types of pancreatic cancer. In addition, people who were diagnosed with ampullary cancer were included in the final data analysis because the surgical treatment and prognosis for ampullary cancer is similar to that of pancreatic cancer (American Cancer Society, 2019). The authors conducted a separate analysis excluding individuals with ampullary cancer (n = 9), which produced the same results. As a result, a total of 57 participants were entered into the final data analysis in this study.
Measures
For data collection, the authors used a structured questionnaire in Chinese that included the following items:
• Demographic and clinical characteristics form
• Fatigue Symptom Inventory (FSI)
• Summary of Diabetes Self-Care Activities (SDSCA)
• European Organisation for Research and Treatment of Cancer QOL Questionnaire–Core 30 (EORTC QLQ-C30)
Demographic and clinical characteristics: The authors documented the following demographic information that was provided by the participants during the interview: age, sex, employment status, and years of education. The following clinical variables were collected from medical charts with the participants’ permission: postoperative time (months), BMI, number of comorbidities, DM duration (months), HbA1c level, Karnofsky Performance Status (KPS) score (Karnofsky et al., 1948), diagnosis, cancer stage, surgery type, smoking status, current chemotherapy treatment, and current DM treatment.
Fatigue Symptom Inventory: Fatigue characteristics were measured by the FSI. This 14-item scale includes four domains: fatigue intensity, fatigue interference, fatigue duration, and fatigue pattern (Hann et al., 2000). The items in the fatigue intensity, fatigue interference, and fatigue duration domains were measured by an 11-point Likert-type scale from 0 (no fatigue at all) to 10 (extreme fatigue). The range for the item “number of days fatigued last week” was from 0 to 7 days. Higher scores indicated higher levels of fatigue. The Chinese version of the FSI demonstrated good convergent and divergent validity in individuals with cancer because it was positively correlated with the Cancer Fatigue Scale (r = 0.62) and was negatively and weakly correlated with the Herth Hope Index (r = –0.18). The scale was also tested in individuals with cancer in Taiwan with high reliability (Cronbach’s alpha = 0.88–0.9) (Shun et al., 2006). In the current study, the Cronbach’s alpha was 0.92.
Summary of Diabetes Self-Care Activities: DM self-care activities were measured by the SDSCA. This scale evaluates five domains of DM self-care activities for the past seven days. The five domains consist of diet (2 items), physical activity (2 items), medication (1 item), self-monitoring of blood glucose (1 item), and foot care (3 items). Higher scores indicate more DM self-care activities for the past week. The SDSCA has demonstrated good construct validity across seven studies (Toobert et al., 2000). The Chinese version of the SDSCA has demonstrated acceptable internal consistency in Taiwanese individuals with DM (Cronbach’s alpha = 0.71) (Chang & Fu, 2004). The Cronbach’s alpha was 0.7 in this study.
EORTC QLQ-C30: QOL was evaluated by the EORTC QLQ-C30 questionnaire, which contained a global health status/QOL scale (2 items), a symptom scale (13 items), and five functional scales (physical functioning [5 items], role functioning [2 items], emotional functioning [4 items], cognitive functioning [2 items], and social functioning [2 items]). For the global health status/QOL subscale, items were measured on a seven-point Likert-type scale from 1 (worst global health status/QOL) to 7 (best global health status/QOL). The symptom scale and functional scales were measured on a four-point Likert-type scale from 1 (not at all) to 4 (very much). All scores were transformed to a scale of 0 to 100 (Fayers et al., 2001), with higher scores on the global health status/QOL scale and the functional scales indicating a higher QOL. Higher scores on the symptom subscales represented higher levels of symptomatology. For the correlation and regression analysis in this study, the authors used the scores from the global health status/QOL subscale as the primary outcome variable. In individuals with cancer, the EORTC QLQ-C30 had good reliability and validity (Niezgoda & Pater, 1993). The Chinese version of the EORTC QLQ-C30 also demonstrated adequate reliability with a Cronbach’s alpha of 0.79 (Chie et al., 2002). The Chinese version of the questionnaire was reported to have a positive correlation with the SF-36® health survey questionnaire (r = 0.7), indicating good convergent validity (Chie et al., 2004). In this study, the Cronbach’s alpha was 0.82.
Data Analysis
The authors used IBM SPSS Statistics, version 24.0, to manage and analyze the data. Data cleaning and tests for assumptions of normality, linearity, and homoscedasticity were performed to ensure that required assumptions were met. Means and standard deviations were presented for continuous variables; counts and percentages were reported for categorical variables. Pearson correlation coefficient and independent t tests were used to examine the relationships among demographics (e.g., age, sex), clinical characteristics (e.g., DM duration, chemotherapy), fatigue characteristics, DM self-care activities, and QOL. A multiple regression analysis was performed to examine the factors related to QOL. The authors selected the variables that were statistically significant in the bivariate correlation analysis (i.e., DM duration, total FSI score, fatigue intensity, fatigue duration, fatigue interference, total SDSCA score, and physical activity) as factors to enter into the multiple regression model. To avoid multicollinearity, scores on the subscales of the FSI and SDSCA (i.e., fatigue intensity, fatigue duration, fatigue interference, and physical activity) were not entered into the model. Therefore, the factors included in the final regression model were DM duration, total SDSCA score, and total FSI score. Age and sex were entered into the model as confounders because they were associated with fatigue and QOL (Didarloo & Alizadeh, 2016; Fritschi & Quinn, 2010; Seo et al., 2015). No evidence of multicollinearity was found between the possible factors (tolerance = 0.971–0.985, variance inflation factor = 1.05–1.3). Because of the exploratory nature of this study and the guidance of the study framework, the factors were treated equally and were entered simultaneously (Polit & Beck, 2017; Warner, 2013). In a simultaneous multiple regression, or standard multiple regression, all variables are entered in a single step, and the effect of each variable can be measured while controlling for other variables (Tabachnick & Fidell, 2007; Warner, 2013). The significance level was set at 0.05.
Results
Participants’ Characteristics and Levels of QOL, Fatigue, and DM Self-Care Activities
On average, the 57 participants were aged 66 years, were mostly female, had 10 years of education, and were mostly unemployed. Most of the participants were diagnosed with pancreatic ductal adenocarcinoma, had stage II cancer, underwent pylorus-preserving PD, were interviewed about 11 months after pancreatic surgery, had had DM for about 48 months, and were undergoing insulin injection treatments (see Table 1). 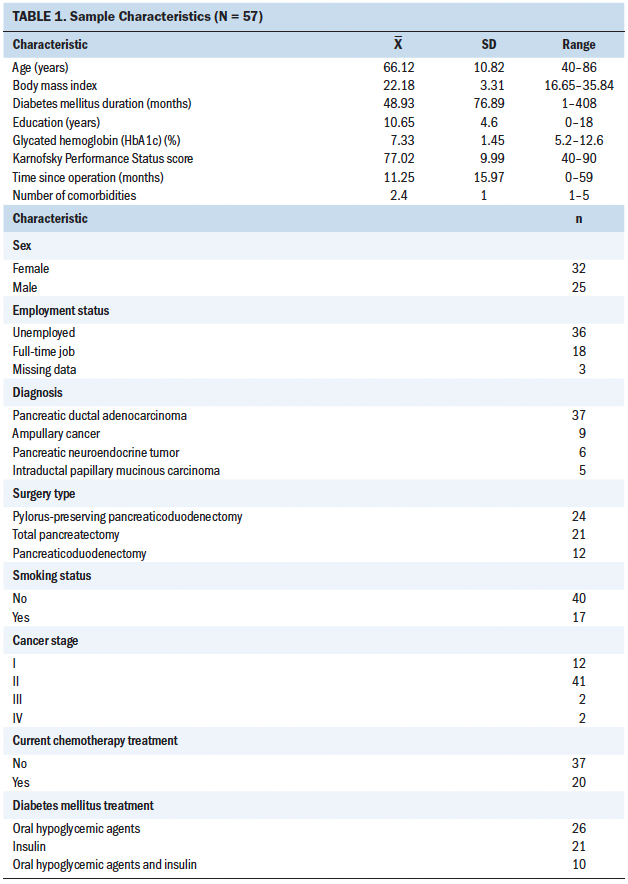
Participants’ scores on the EORTC QLQ-C30, FSI, and SDSCA are shown in Table 2. The participants reported a moderate overall QOL (mean = 53.8, SD = 20.8). On average, the participants scored high on the functional scales; the mean scores for physical, role, emotional, cognitive, and social functioning were 83 (SD = 23.5), 78.9 (SD = 28.4), 81.3 (SD = 21.3), 75.7 (SD = 22.9), and 77.8 (SD = 28.2), respectively. For the symptom subscales of the EORTC QLQ-C30, the participants were most likely to report insomnia (mean = 27.5, SD = 35.1) and fatigue (mean = 27.1, SD = 23.8). According to the FSI, the mean of the total fatigue score was 24.5 (SD = 22.3). The participants were experiencing a mild intensity of fatigue (mean = 3.1, SD = 3), were having fatigue about three days per week (mean = 2.9, SD = 2.8), and had a low level of fatigue interference (mean = 1.5, SD = 2). For the SDSCA, the mean scores of the total SDSCA score, diet, physical activity, foot care, medication, and self-monitoring of blood glucose were 31.3 (SD = 13.2), 3 (SD = 1.4), 4.1 (SD = 2.9), 2.2 (SD = 3.1), 6.7 (SD = 1), and 4.1 (SD = 3.3), respectively. The participants were more likely to take medications and least likely to engage in foot care. 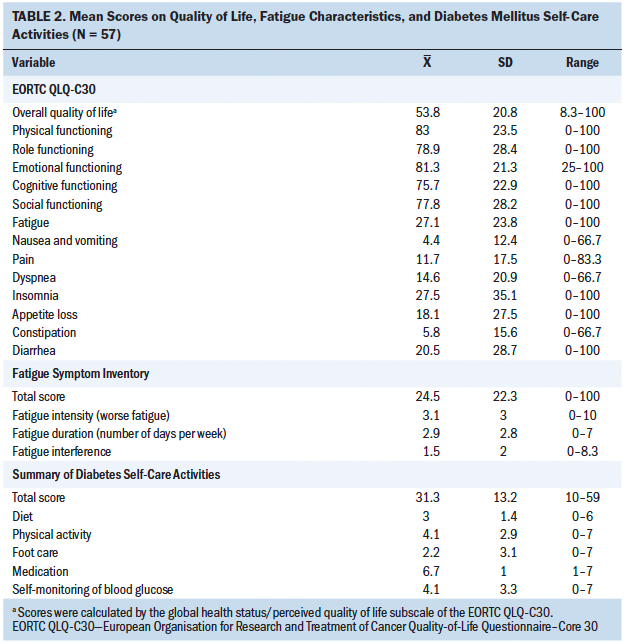
Correlations Among Demographic and Clinical Characteristics, Fatigue, DM Self-Care Activities, and QOL
Table 3 shows the correlations among demographic and clinical characteristics, fatigue, DM self-care activities, and QOL. Longer DM duration was statistically significantly correlated with higher QOL (r = 0.3, p = 0.026). Other demographic and clinical variables were not statistically significantly correlated with QOL. A higher total FSI score (r = –0.5, p < 0.001), fatigue intensity (r = –0.47, p < 0.001), fatigue duration (r = –0.42, p = 0.001), and fatigue interference (r = –0.46, p < 0.001) were statistically significantly correlated with lower QOL. A higher total SDSCA score (r = 0.31, p = 0.021) and higher physical activity (r = 0.3, p = 0.025) were statistically significantly correlated with better QOL. Diet, foot care, medication, and blood glucose monitoring were not statistically significantly associated with QOL. 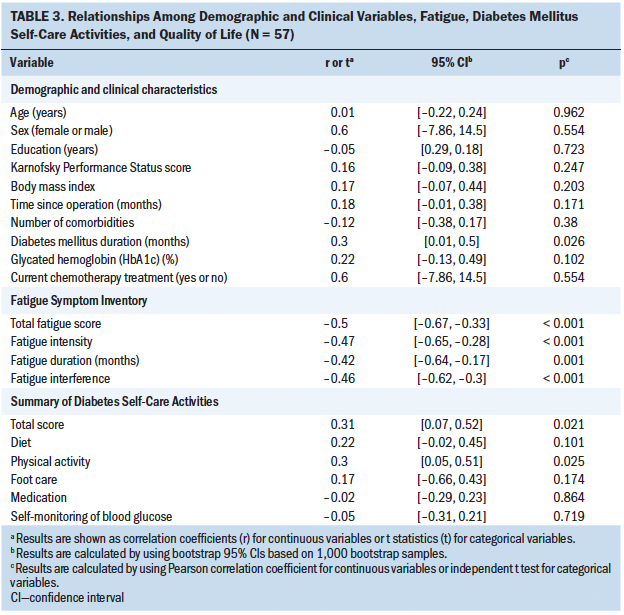
Determinants of QOL
Variables significantly correlated with QOL (i.e., DM duration, total FSI score, and total SDSCA score) and possible confounders (i.e., age and sex) were entered into the multiple regression model (see Table 4). The results showed that DM duration (b = 0.28, t = 2.51, p = 0.015), total FSI score (b = –0.48, t = –4.3, p < 0.001), and total SDSCA score (b = 0.23, t = 2.08, p = 0.043) were statistically significant factors related to QOL. The variables in the model explained 39% (adjusted R2 = 33%) of the variances in QOL. 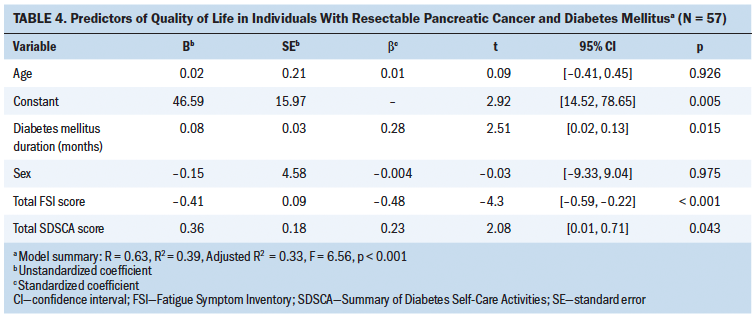
Discussion
This study showed that, in individuals with a dual diagnosis of resectable pancreatic cancer and DM, shorter duration of DM and higher levels of fatigue (i.e., fatigue intensity, fatigue duration, and fatigue interference) were correlated with lower QOL; higher scores in DM self-care activities and physical activity were correlated with higher QOL. Of note, total fatigue scores, DM self-care activities, and DM duration were significant determinants of QOL in this sample.
In this study, individuals with resectable pancreatic cancer and DM scored a mean of 53.8 (SD = 20.8) in QOL 11 months after pancreatic surgery, which is lower than the QOL reported in a study of 68 people 12 months after pancreatic resection for malignancy (mean = 74, SD = 16) (Heerkens et al., 2016). Although this was a cross-sectional study where the temporal and causal relationship between cancer and DM could not be determined, it is possible that the burden of pancreatic cancer, its surgical treatment, DM, and DM-related treatments all contributed to the lower QOL in this population (Bauer et al., 2018; Scollan-Koliopoulos et al., 2013). The results also showed that more interventions in this population are needed to improve their QOL.
Unlike previous studies in individuals with type 2 DM that found shorter duration of DM to be related to better QOL (Didarloo & Alizadeh, 2016; Wan et al., 2016), shorter DM duration was correlated with worse QOL in this study. The possible reason for this inconsistency might be because of the co-occurrence of pancreatic cancer and DM. Individuals with pancreatic cancer have worse QOL at one month post–pancreatic resection, and their QOL steadily recovers at six months after surgery (Heerkens et al., 2016). In addition, newly developed DM secondary to surgery (e.g., total pancreatectomy) has a negative impact on QOL (Belyaev et al., 2013). A previous study showed that, as compared to long-term DM in individuals with pancreatic cancer, new-onset DM was associated with worse clinical outcomes, including larger tumor size and higher BMI, suggesting a higher disease burden in individuals with pancreatic cancer who had a more recent diagnosis of DM (Li et al., 2015). Therefore, the concurrent burden of pancreatic cancer (including its related treatments, such as surgery) and DM might be a possible explanation for the association between shorter DM duration and lower QOL in the current study.
In the current study, higher fatigue intensity, fatigue duration, and fatigue interference were all correlated with lower QOL. However, in a study by Sun and Lin (2015) with 187 individuals with various types of cancer, the relationship between fatigue interference and QOL was not statistically significant. The overlapping disease mechanisms of cancer and DM might be a possible reason for this inconsistency. In the cancer population, hypothalamic–pituitary–adrenal (HPA) axis dysfunction (e.g., reduced morning serum cortisol levels) and circadian cycle disruption (e.g., flattened diurnal cortisol slopes) were proposed as possible mechanisms of cancer-related fatigue (Ryan et al., 2007). Similarly, a flattened circadian cortisol rhythm and a trend of blunted cortisol-awakening response were also found in the population of individuals with DM (Joseph & Golden, 2017; Lederbogen et al., 2011). Because cancer and DM are related to the disturbance of the HPA axis and the circadian cycle (Joseph & Golden, 2017; Ryan et al., 2007), participants in this study might have longer fatigue duration and worse fatigue interference and, therefore, lower QOL than individuals with cancer but without DM. Another possible explanation for the inconsistent findings was that different instruments were used to measure fatigue. In the study by Sun and Lin (2015), the Brief Fatigue Inventory (BFI) that measured fatigue for the past 24 hours was used. In the current study, the FSI that measured fatigue for the past week was used. In other words, compared to the BFI, the FSI might have captured the long-term impact of fatigue, which led to a different finding in the relationship between fatigue interference and QOL.
This study also showed that the frequency of DM self-care activities was a significant factor related to QOL, which is similar to the findings in a recent study in individuals with cancer and DM in an outpatient setting that reported that individuals who were in a DM self-care education program performed more DM self-care activities and had better QOL (Al-Taie et al., 2020). In this study, among the five domains of DM self-care activities, physical activity is significantly correlated with QOL in individuals with pancreatic cancer and DM, which is consistent with previous studies in individuals with DM (Imayama et al., 2011; Wan et al., 2016). Similar to a previous study in individuals with DM (Lin et al., 2016), physical activity was less frequently performed in individuals with pancreatic cancer and DM as compared to medication adherence. Participants in the current study reported higher scores in physical activity (mean = 4.1, SD = 2.9) than individuals with type 1 DM (mean = 3.1, SD = 2.4) (Lin et al., 2016). However, this result did not represent that the participants in this study had performed an adequate amount of physical activity.
In the multiple regression analysis, DM duration, total FSI score, and total SDSCA score were significant determinants of QOL. This result emphasizes the importance of an in-depth evaluation of all characteristics of fatigue and all aspects of DM self-care activities in individuals with resectable pancreatic cancer and DM. Previous studies in the cancer population supported this finding, indicating that healthcare providers should assess and integrate the severity, frequency, and interference of fatigue to help identify, treat, and lower the impact of fatigue in individuals with cancer (Berger et al., 2015; Wang & Woodruff, 2015). Regarding DM self-care activities, previous studies also supported that a higher total score of SDSCA (i.e., diet, physical activity, medication, self-monitoring of blood glucose, and foot care) is significantly associated with higher QOL in individuals with DM (Jannoo et al., 2017; Marinho et al., 2018).
Limitations
This study had limitations that should be considered when interpreting the results. First, because this was a single-center study with a small sample size, the results might be limited in generalizability to all individuals with resectable pancreatic cancer and DM. In addition, to identify potential factors to enter into the regression analysis, the correlation calculations were conducted multiple times using a small sample size, which could increase the risk of a type 1 error. In addition, this was a study using a cross-sectional, observational design; therefore, causal relationships among fatigue, DM self-care activities, and QOL cannot be established. Lastly, the DM self-care activities measured by the SDSCA only measured whether the participants had done a certain activity during the past week, which might not have captured all of the essential information related to DM self-management. For example, more specific information on physical activity (e.g., exercise type, exercise intensity, exercise duration) should be evaluated to know if participants had an adequate amount of physical activity per week.
Implications for Nursing
Previous studies have shown that individuals with cancer and DM tend to prioritize their cancer treatment over DM self-management (Hershey et al., 2012). This study adds to the literature that nurses should assist individuals with pancreatic cancer and DM in integrating DM self-management into their daily routines to improve QOL. According to the current study’s results, nurses who care for individuals with resectable pancreatic cancer and DM play a crucial role in evaluating patients recently diagnosed with DM so that they can intervene promptly to ensure optimal QOL in this population. Nurses should provide a comprehensive evaluation of fatigue (i.e., fatigue intensity, fatigue duration, and fatigue interference) and DM self-care activities (i.e., physical activity, diet, medication, self-monitoring of blood glucose, and foot care) and provide patient education accordingly. For example, incorporating physical activity according to the patients’ preferences will likely improve their glycemic control (Colberg et al., 2016), decrease fatigue (Witlox et al., 2018), and improve QOL.
Future studies with a larger sample size should focus on developing and testing integrated interventions that include fatigue management and DM self-care to improve QOL in individuals with resectable pancreatic cancer and DM. A more in-depth investigation of physical activity, such as the measurement of physical activity duration, types, and barriers, is recommended to help improve the QOL in this population. Future research with a longitudinal design to examine the trajectory of fatigue, DM self-care activities, and QOL is also suggested. Because previous studies suggested overlapping disease mechanisms of pancreatic cancer and DM (e.g., cortisol levels and circadian rhythm), research should also investigate the underlying mechanisms for fatigue in individuals with concurrent pancreatic cancer and DM. Another area that requires future research is the development of theories addressing symptoms, DM self-management, and QOL for individuals with a dual diagnosis of cancer and DM. 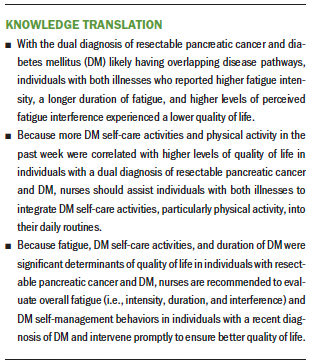
Conclusion
Individuals with a dual diagnosis of resectable pancreatic cancer and DM have to face the impact of both illnesses, their subsequent treatments, and symptom burdens, resulting in worse QOL. This study showed that a shorter duration of DM, higher fatigue, and lower levels of DM self-care activities were determinants of worse QOL in individuals with resectable pancreatic cancer and DM. The current study also emphasized the importance of evaluating all characteristics of fatigue (i.e., fatigue intensity, fatigue duration, and fatigue interference) and DM self-care (i.e., physical activity, diet, medication, self-monitoring of blood glucose, and foot care) so that nurses could provide interventions to improve the individuals’ QOL. Future studies with a larger sample size focusing on the development of integrated interventions addressing fatigue and DM self-management will help improve the QOL in this population.
About the Author(s)
Hsuan-Ju Kuo, MSN, RN, is a student and Nien-Tzu Chang, RN, PhD, is an associate professor, both in the School of Nursing in the College of Medicine at National Taiwan University in Taipei; Yu-Wen Tien, MD, PhD, is a professor in the Department of Surgery at National Taiwan University Hospital in Taipei; Yun-Jen Chou, RN, PhD, is a postdoctoral fellow in the School of Nursing at National Taiwan University; and Shiow-Ching Shun, RN, PhD, is a professor in the School of Nursing in the College of Medicine at National Taiwan University. No financial relationships to disclose. Kuo, Chang, Tien, and Shun contributed to the conceptualization and design. Kuo and Shun completed the data collection and provided the analysis. Kuo, Chang, Chou, and Shun provided statistical support. Kuo, Chou, and Shun contributed to the manuscript preparation. Shun can be reached at scshun@ntu.edu.tw, with copy to ONFEditor@ons.org. (Submitted November 2020. Accepted January 6, 2021.)
References
Adriaanse, M.C., Drewes, H.W., van der Heide, I., Struijs, J.N., & Baan, C.A. (2016). The impact of comorbid chronic conditions on quality of life in type 2 diabetes patients. Quality of Life Research, 25(1), 175–182. https://doi.org/10.1007/s11136-015-1061-0
Aggarwal, G., Kamada, P., & Chari, S.T. (2013). Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas, 42(2), 198–201. https://doi.org/10.1097/MPA.0b013e3182592c96
Al-Taie, A., Izzettin, F.V., Sancar, M., & Köseoğlu, A. (2020). Impact of clinical pharmacy recommendations and patient counselling program among patients with diabetes and cancer in outpatient oncology setting. European Journal of Cancer Care, 29(5), e13261. https://doi.org/10.1111/ecc.13261
American Cancer Society. (2019). What is pancreatic cancer? https://www.cancer.org/cancer/pancreatic-cancer/about/what-is-pancreati…
American Diabetes Association. (2020). Introduction: Standards of medical care in diabetes—2020. Diabetes Care, 43(Suppl. 1), S1–S2. https://doi.org/10.2337/dc20-Sint
Bauer, M.R., Bright, E.E., MacDonald, J.J., Cleary, E.H., Hines, O.J., & Stanton, A.L. (2018). Quality of life in patients with pancreatic cancer and their caregivers: A systematic review. Pancreas, 47(4), 368–375. https://doi.org/10.1097/MPA.0000000000001025
Beger, H.G., Poch, B., Mayer, B., & Siech, M. (2018). New onset of diabetes and pancreatic exocrine insufficiency after pancreaticoduodenectomy for benign and malignant tumors: A systematic review and meta-analysis of long-term results. Annals of Surgery, 267(2), 259–270. https://doi.org/10.1097/SLA.0000000000002422
Beijer, S., Kempen, G.I.J.M., Pijls-Johannesma, M.C., de Graeff, A., & Dagnelie, P.C. (2008). Determinants of overall quality of life in preterminal cancer patients. International Journal of Cancer, 123(1), 232–235. https://doi.org/10.1002/ijc.23497
Belyaev, O., Herzog, T., Chromik, A.M., Meurer, K., & Uhl, W. (2013). Early and late postoperative changes in the quality of life after pancreatic surgery. Langenbeck’s Archives of Surgery, 398(4), 547–555. https://doi.org/10.1007/s00423-013-1076-3
Berger, A.M., Mitchell, S.A., Jacobsen, P.B., & Pirl, W.F. (2015). Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA: A Cancer Journal for Clinicians, 65(3), 190–211. https://doi.org/10.3322/caac.21268
Chang, S.C., & Fu, C.C. (2004). Patient outcomes and their predictive factors of diabetes care in Hualien. Tzu Chi Nursing Journal, 3(4), 38–48. https://doi.org/10.6974/TCNJb.200412.0038
Chie, W.C., Yang, C.H., Hsu, C., & Lai, C.C. (2002). Introduction of the EORTC disease-specific quality of life questionnaires for cancer patients. Formosan Journal of Medicine, 6(2), 220–227. https://doi.org/10.6320/FJM.2002.6(2).13
Chie, W.C., Yang, C.H., Hsu, C., & Yang, P.C. (2004). Quality of life of lung cancer patients: Validation of the Taiwan Chinese version of the EORTC QLQ-C30 and QLQ-LC13. Quality of Life Research, 13(1), 257–262. https://doi.org/10.1023/b:qure.0000015295.74812.06
Cloyd, J.M., Tran Cao, H.S., Petzel, M.Q.B., Denbo, J.W., Parker, N.H., Nogueras-González, G.M., . . . Katz, M.H.G. (2017). Impact of pancreatectomy on long-term patient-reported symptoms and quality of life in recurrence-free survivors of pancreatic and periampullary neoplasms. Journal of Surgical Oncology, 115(2), 144–150. https://doi.org/10.1002/jso.24499
Colberg, S.R., Sigal, R.J., Yardley, J.E., Riddell, M.C., Dunstan, D.W., Dempsey, P.C., . . . Tate, D.F. (2016). Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39(11), 2065–2079. https://doi.org/10.2337/dc16-1728
Didarloo, A., & Alizadeh, M. (2016). Health-related quality of life and its determinants among women with diabetes mellitus: A cross-sectional analysis. Nursing and Midwifery Studies, 5(1), e28937. https://doi.org/10.17795/nmsjournal28937
Duan, W., Shen, X., Lei, L., Xu, Q., Yu, Y., Li, R., . . . Ma, Q. (2014). Hyperglycemia, a neglected factor during cancer progression. BioMed Research International, 2014, e28937. https://doi.org/10.1155/2014/461917
Eom, K., Chie, E.K., Kim, K., Jang, J.J., Kim, S.W., Oh, D.Y., . . . Ha, S.W. (2013). Postoperative chemoradiotherapy following pancreaticoduodenectomy: Impact of dose-volumetric parameters on the development of diabetes mellitus. Strahlentherapie und Onkologie, 189(9), 753–758. https://doi.org/10.1007/s00066-013-0405-3
Fayers, P., Aaronson, N.K., Bjordal, K., Groenvold, M., Curran, D., & Bottomley, A. (2001). EORTC QLQ-C30 scoring manual (3rd ed.). European Organisation for Research and Treatment of Cancer.
Finsterer, J.M., & Mahjoub, S.Z. (2014). Fatigue in healthy and diseased individuals. American Journal of Hospice and Palliative Medicine, 31(5), 562–575. https://doi.org/10.1177/1049909113494748
Fritschi, C., & Quinn, L. (2010). Fatigue in patients with diabetes: A review. Journal of Psychosomatic Research, 69(1), 33–41. https://doi.org/10.1016/j.jpsychores.2010.01.021
Fong, Z.V., Alvino, D.M., Fernández-del Castillo, C., Nipp, R.D., Traeger, L.N., Ruddy, M., . . . Ferrone, C.R. (2017). Health-related quality of life and functional outcomes in 5-year survivors after pancreaticoduodenectomy. Annals of Surgery, 266(4), 685–692. https://doi.org/10.1097/SLA.0000000000002380
García, A.A., Brown, S.A., Horner, S.D., Zuñiga, J., & Arheart, K.L. (2015). Home-based diabetes symptom self-management education for Mexican Americans with type 2 diabetes. Health Education Research, 30(3), 484–496. https://doi.org/10.1093/her/cyv018
Goedendorp, M.M., Tack, C.J., Steggink, E., Bloot, L., Bazelmans, E., & Knoop, H. (2014). Chronic fatigue in type 1 diabetes: Highly prevalent but not explained by hyperglycemia or glucose variability. Diabetes Care, 37(1), 73–80. https://doi.org/10.2337/dc13-0515/-/DC1
Gustavell, T., Sundberg, K., & Langius-Eklöf, A. (2020). Using an interactive app for symptom reporting and management following pancreatic cancer surgery to facilitate person-centered care: Descriptive study. JMIR mHealth and uHealth, 8(6), e17855. https://doi.org/10.2196/17855
Hammer, M., Storey, S., Hershey, D.S., Brady, V.J., Davis, E., Mandolfo, N., . . . Olausson, J. (2019). Hyperglycemia and cancer: A state-of-the-science review. Oncology Nursing Forum, 46(4), 459–472. https://doi.org/10.1188/19.ONF.459-472
Hann, D.M., Denniston, M.M., & Baker, F. (2000). Measurement of fatigue in cancer patients: Further validation of the Fatigue Symptom Inventory. Quality of Life Research, 9(7), 847–854. https://doi.org/10.1023/A:1008900413113
Heerkens, H.D., Tseng, D.S.J., Lips, I.M., van Santvoort, H.C., Vriens, M.R., Hagendoorn, G.J., . . . Molenaar, I.Q. (2016). Health-related quality of life after pancreatic resection for malignancy. British Journal of Surgery, 103(3), 257–266. https://doi.org/10.1002/bjs.10032
Hershey, D.S., Tipton, J., Given, B., & Davis, E. (2012). Perceived impact of cancer treatment on diabetes self-management. Diabetes Educator, 38(6), 779–790. https://doi.org/10.1177/0145721712458835
Imayama, I., Plotnikoff, R.C., Courneya, K.S., & Johnson, J.A. (2011). Determinants of quality of life in type 2 diabetes population: The inclusion of personality. Quality of Life Research, 20(1), 551–558. https://doi.org/10.1007/s11136-010-9772-8
Jannoo, Z., Wah, Y.B., Lazim, A.M., & Hassali, M.A. (2017). Examining diabetes distress, medication adherence, diabetes self-care activities, diabetes-specific quality of life and health-related quality of life among type 2 diabetes mellitus patients. Journal of Clinical and Translational Endocrinology, 9, 48–54. https://doi.org/10.1016/j.jcte.2017.07.003
Jing, X., Chen, J., Dong, Y., Han, D., Zhao, H., Wang, X., . . . Ma, J. (2018). Related factors of quality of life of type 2 diabetes patients: A systematic review and meta-analysis. Health and Quality of Life Outcomes, 16(1), 189. https://doi.org/10.1186/s12955-018-1021-9
Joseph, J.J., & Golden, S.H. (2017). Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Annals of the New York Academy of Sciences, 1391(1), 20–34. https://doi.org/10.1111/nyas.13217
Juel, C.T.B., Dejgaard, T.F., Hansen, C.P., Storkholm, J.H., Vilsbøll, T., Lund, A., & Knop, F.K. (2021). Glycemic control and variability of diabetes secondary to total pancreatectomy assessed by continuous glucose monitoring. Journal of Clinical Endocrinology and Metabolism, 106(1), 168–173. https://doi.org/10.1210/clinem/dgaa731
Karnofsky, D.A., Abelmann, W.H., Craver, L.F., & Burchenal, J.H. (1948). The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer, 1(4), 634–656. https://doi.org/10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3…
Kuo, H.J., Tien, Y.W., Chang, N.T., Chou, Y.J., & Shun, S.C. (2019). Comparison of fatigue and quality of life in individuals with pancreatogenic diabetes after total or partial pancreatectomy. Oncology Nursing Forum, 46(5), E159–E170. https://doi.org/10.1188/19.ONF.E159-E170
Lederbogen, F., Hummel, J., Fademrecht, C., Krumm, B., Kühner, C., Deuschle, M., . . . Breivogel, B. (2011). Flattened circadian cortisol rhythm in type 2 diabetes. Experimental and Clinical Endocrinology and Diabetes, 119(9), 573–575. https://doi.org/10.1055/s-0031-1275288
Lewis, A.R., Pihlak, R., & McNamara, M.G. (2018). The importance of quality-of-life management in patients with advanced pancreatic ductal adenocarcinoma. Current Problems in Cancer, 42(1), 26–39. https://doi.org/10.1016/j.currproblcancer.2018.01.013
Li, D., Mao, Y., Chang, P., Liu, C., Hassan, M.M., Yeung, S.J., & Abbruzzese, J.L. (2015). Impacts of new-onset and long-term diabetes on clinical outcome of pancreatic cancer. American Journal of Cancer Research, 5(10), 3260–3269.
Li, H., Ji, M., Scott, P., & Dunbar-Jacob, J.M. (2019). The effect of symptom clusters on quality of life among patients with type 2 diabetes. Diabetes Educator, 45(3), 287–294. https://doi.org/10.1177/0145721719837902
Lin, K., Yang, X., Yin, G., & Lin, S. (2016). Diabetes self-care activities and health-related quality-of-life of individuals with type 1 diabetes mellitus in Shantou, China. Journal of International Medical Research, 44(1), 147–156. https://doi.org/10.1177/0300060515597933
Maeda, H., & Hanazaki, K. (2011). Pancreatogenic diabetes after pancreatic resection. Pancreatology, 11(2), 268–276. https://doi.org/10.1159/000328785
Marinho, F.S., Moram, C.B.M., Rodrigues, P.C., Leite, N.C., Salles, G.F., & Cardoso, C.R.L. (2018). Treatment adherence and its associated factors in patients with type 2 diabetes: Results from the Rio de Janeiro Type 2 Diabetes Cohort Study. Journal of Diabetes Research, 2018, 8970196. https://doi.org/10.1155/2018/8970196
Nielsen, H.B., Ovesen, L.L., Mortensen, L.H., Lau, C.J., & Joensen, L.E. (2016). Type 1 diabetes, quality of life, occupational status and education level—A comparative population-based study. Diabetes Research and Clinical Practice, 121, 62–68. https://doi.org/10.1016/j.diabres.2016.08.021
Niezgoda, H.E., & Pater, J.L. (1993). A validation study of the domains of the core EORTC quality of life questionnaire. Quality of Life Research, 2(5), 319–325. https://doi.org/10.1007/bf00449426
Park, H., Park, C., Quinn, L., & Fritschi, C. (2015). Glucose control and fatigue in type 2 diabetes: The mediating roles of diabetes symptoms and distress. Journal of Advanced Nursing, 71(7), 1650–1660. https://doi.org/10.1111/jan.12632
Polit, D.F., & Beck, C.T. (2017). Nursing research: Generating and assessing evidence for nursing practice (10th ed.). Wolters Kluwer.
Puckett, Y., & Garfield, K. (2020). Pancreatic cancer. StatPearls. https://www.statpearls.com/ArticleLibrary/viewarticle/26569
Ryan, J.L., Carroll, J.K., Ryan, E.P., Mustian, K.M., Fiscella, K., & Morrow, G.R. (2007). Mechanisms of cancer-related fatigue. Oncologist, 12(Suppl. 1), 22–34. https://doi.org/10.1634/theoncologist.12-S1-22
Scollan-Koliopoulos, M., Bleich, D., Rapp, K.J., Wong, P., Hofmann, C.J., & Raghuwanshi, M. (2013). Health-related quality of life, disease severity, and anticipated trajectory of diabetes. Diabetes Educator, 39(1), 83–91. https://doi.org/10.1177/0145721712467697
Scott, J.A., Lasch, K.E., Barsevick, A.M., & Piault-Louis, E. (2011). Patients’ experiences with cancer-related fatigue: A review and synthesis of qualitative research. Oncology Nursing Forum, 38(3), E191–W203. https://doi.org/10.1188/11.ONF.E191-E203
Seo, Y.M., Hahm, J.R., Kim, T.K., & Choi, W.H. (2015). Factors affecting fatigue in patients with type II diabetes mellitus in Korea. Asian Nursing Research, 9(1), 60–64. https://doi.org/10.1016/j.anr.2014.09.004
Shim, Y.T., Lee, J., Toh, M.P.H.S., Tang, W.E., & Ko, Y. (2012). Health-related quality of life and glycaemic control in patients with type 2 diabetes mellitus in Singapore. Diabetic Medicine, 29(8), e241–e248. https://doi.org/10.1111/j.1464-5491.2012.03689.x
Shun, S.C., Beck, S.L., Pett, M.A., & Berry, P.H. (2006). Psychometric testing of three Chinese fatigue instruments in Taiwan. Journal of Pain and Symptom Management, 32(2), 155–167. https://doi.org/10.1016/j.jpainsymman.2006.02.011
Sun, J.L., & Lin, C.C. (2015). Relationships among daytime napping and fatigue, sleep quality, and quality of life in cancer patients. Cancer Nursing, 39(5), 383–392. https://doi.org/10.1097/NCC.0000000000000299
Tabachnick, B.G., & Fidell, L.S. (2007). Using multivariate statistics (5th ed.). Pearson Education.
Toobert, D.J., Hampson, S.E., & Glasgow, R.E. (2000). The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care, 23(7), 943–950. https://doi.org/10.2337/diacare.23.7.943
Velanovich, V., & Wollner, I. (2011). Quality of life and performance status in patients with pancreatic and periampullary tumors. International Journal of Clinical Oncology, 16(4), 401–407. https://doi.org/10.1007/s10147-011-0200-z
Vissers, P.A.J., Falzon, L., van de Poll-Franse, L., Pouwer, F., & Thong, M.S.Y. (2016). The impact of having both cancer and diabetes on patient-reported outcomes: A systematic review and directions for future research. Journal of Cancer Survivorship, 10(2), 406–415. https://doi.org/10.1007/s11764-015-0486-3
Wan, E.Y.F., Fung, C.S.C., Choi, E.P.H., Wong, C.K.H., Chan, A.K.C., Chan, K.H.Y., & Lam, C.L.K. (2016). Main predictors in health-related quality of life in Chinese patients with type 2 diabetes mellitus. Quality of Life Research, 25(11), 2957–2965. https://doi.org/10.1007/s11136-016-1324-4
Wang, X.S., & Woodruff, J.F. (2015). Cancer-related and treatment-related fatigue. Gynecologic Oncology, 136(3), 446–452. https://doi.org/10.1016/j.ygyno.2014.10.013
Warner, R.M. (2013). Applied statistics: From bivariate through multivariate techniques (2nd ed.). Sage.
Witlox, L., Hiensch, A.E., Velthuis, M.J., Steins Bisschop, C.N., Los, M., Erdkamp, F.L.G., . . . May, A.M. (2018). Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Medicine, 16(1), 86. https://doi.org/10.1186/s12916-018-1075-x
Wong, S.S., Hsu, F.C., Avis, N.E., & Clark, C.J. (2020). Health-related quality of life and medical comorbidities in older patients with pancreatic adenocarcinoma: An analysis using the 1998–2011 Surveillance, Epidemiology, and End Results–Medicare health outcomes survey data. Journal of Geriatric Oncology, 11(4), 633–639. https://doi.org/10.1016/j.jgo.2019.08.010
World Health Organization. (2020). Cancer: Key statistics. https://www.who.int/cancer/resources/keyfacts/en
World Health Organization. (2021). Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes
Yao, G. (2002). Development and applications of the WHOQOL-Taiwan version. Formosan Journal of Medicine, 6(2), 193–200.
Zhang, Q., Zeng, L., Chen, Y., Lian, G., Qian, C., Chen, S., . . . Huang, K. (2016). Pancreatic cancer epidemiology, detection, and management. Gastroenterology Research and Practice, 2016, 1–10. https://doi.org/10.1155/2016/8962321




