Nurse-Delivered Telephone Intervention to Reduce Oral Mucositis and Prevent Dehydration
Problem Statement: This study evaluates the feasibility of a nurse-delivered telephone intervention to reduce oral mucositis severity and prevent dehydration in patients with lung or head and neck cancer undergoing chemotherapy and radiation therapy.
Design: This study used a two-phase, qualitatively driven, mixed-methods descriptive design.
Data Sources: 11 participants were recruited from an academic cancer center in southern Florida. Participants received symptom management education followed by twice-weekly tailored nurse coaching telephone calls.
Analysis: Questionnaires measuring symptom severity, health-related quality of life, perceived self-efficacy, and symptom self-management were administered at four data points. Data on unscheduled medical visits were collected. Guided interviews were conducted four weeks post-treatment and analyzed qualitatively using content analysis.
Findings: Participants found the intervention to be acceptable. Oral mucositis symptom severity was minimized, and dehydration was avoided. The intervention enabled symptom self-management and improved perceived self-efficacy.
Implications for Practice: Emotional support provided by the nurse was crucial, exemplifying improvement over an automated system.
Jump to a section
Patients with cancer receiving chemotherapy and radiation therapy experience multiple co-occurring symptom clusters from side effects of treatment (Honea et al., 2007). Oral mucositis is a frequent symptom experienced within a symptom cluster by a significant majority of patients with lung or head and neck cancer (Bar-Ad et al., 2014; Elting et al., 2008). Oral mucositis is an acute, painful condition precipitated by symptoms of a sore mouth or sore throat, leading to difficulty swallowing or speaking, mood disturbances, and other symptoms. If left untreated, patients can become dehydrated and malnourished, resulting in treatment delays, inadequate cancer treatment, and, ultimately, a poorer prognosis (Arrieta et al., 2013; Deek et al., 2016). Uncontrolled oral mucositis symptoms worsen overall symptom severity and negatively affect a patient’s health-related quality of life (HRQOL) (Bar-Ad et al., 2014; Elting et al., 2008). Patients with lung or head and neck cancer are the most frequent visitors to emergency departments and urgent care centers seeking symptom relief (Barbera et al., 2010, 2013; Eskander et al., 2018; Mayer et al., 2011; Ruegg, 2013).
Anxiety and depression coupled with symptom severity and distress are found more often in patients with lung or head and neck cancer than in other patients with cancer (Buchanan et al., 2010; Liao et al., 2011; Mehnert et al., 2014; Salvo et al., 2012; Zabora et al., 2001), resulting in reduced symptom management, decreased HRQOL, and twice the risk of an accelerated death than patients not experiencing anxiety and depression (Arrieta et al., 2013; Chen et al., 2011). Patients with oral mucositis also experience increased depressed mood as a correlative symptom while undergoing chemotherapy and radiation therapy (Mason et al., 2016). In addition, these patients have high levels of supportive care needs in health information and communication, leading to increased psychological stress, unmet needs, and inferior HRQOL post-treatment (Buchanan et al., 2010; Liao et al., 2011; Llewellyn et al., 2006; Ugalde et al., 2011; Walling et al., 2016).
Cancer symptom management intervention research explicitly designed for reducing overall symptom severity in patients receiving chemotherapy and radiation therapy is limited (Sanson-Fisher et al., 2010). Most studies addressing the treatment of oral mucositis symptoms have included only patients with head and neck cancer and have involved a one-size-fits-all approach (Carey et al., 2012; Mason et al., 2016; Moslemi et al. 2016; Ray-Chaudhuri et al., 2013). However, variance in symptom experience, type of disease, and treatment-related symptom severity necessitates a tailored approach (Gao & Yuan, 2011; Kirkova et al., 2010; Zabora et al., 2001).
The spectrum of supportive care research involving symptom management targeted at controlling oral mucositis has included interventions such as medication, intermittently monitored home-based or clinic nurse visits, nurse-delivered telecommunication systems, and automated telemonitoring systems (Eilers et al., 2014; Kartin et al., 2014; Mooney et al., 2017; Rubenstein et al., 2004; Skrutkowski et al., 2008). Of note, there are no recommended evidence-based guidelines to prevent oral mucositis and other symptoms in patients undergoing chemotherapy and radiation therapy (Riley et al.; 2017; Worthington et al., 2007). Systematic reviews by Carey et al. (2012) and Moslemi et al. (2016) concluded that, although intervention studies did meet some supportive care needs of patients with cancer, results were inconsistent and lacked support for one intervention approach over another.
Several nurse-delivered coaching interventions have shown promise in helping patients with cancer with improved symptom management, HRQOL, and vitality during treatment (Badger et al., 2013; Howell et al., 2017; Suh & Lee, 2017). One of the most promising nursing interventions to improve patient management of oral mucositis and other distressing symptoms derives from the PRO-SELF© program (Dodd & Miaskowski, 2000; Larson et al., 1998). The premise for the program is that, after a supportive relationship (based on the nurse’s own style of patient interaction) is established with the patient, the nurse provides knowledge and teaches skills patients need to be successful in managing their oral mucositis symptoms at home.
To date, published research urges the exploration of innovative supportive care interventions to lessen the severity and consequences of oral mucositis for patients with lung or head and neck cancer undergoing concurrent chemotherapy and radiation therapy (da Cruz Campos et al., 2014; Mason et al., 2016; Moslemi et al., 2016; Nonzee et al., 2008; Worthington et al., 2007). Despite these recommendations, there is a lack of published studies focusing on controlling oral mucositis severity to prevent dehydration.
The primary purpose of the current study was to determine if a tailored nurse coaching intervention could be feasibly delivered via telephone and acceptable to patients with lung or head and neck cancer during chemotherapy and radiation therapy to reduce oral mucositis severity and prevent dehydration. Secondary purposes were investigated to test the intervention’s effects on behavioral, physiologic, and psychological outcomes. These outcomes encompassed overall symptom severity, HRQOL, unscheduled medical visits, and exploratory outcomes of perceived self-efficacy and symptom self-management.
This study was guided by the theory of symptom self-management (Hoffman, 2013) that posits that as symptom severity rises, patients will increase (or at least stabilize) their perceived self-efficacy to act on severity to self-manage their symptoms to feel better.
Methods
Intervention
The nurse-delivered telephone intervention consisted of symptom management education and nurse coaching delivered via a proactive approach twice weekly to patients with lung or head and neck cancer by an oncology-experienced RN trained in helping patients manage their symptoms caused by cancer treatment. The symptom management education and nurse coaching intervention was modeled, in part, after the PRO-SELF program for cancer symptom self-management (West et al., 2003) (see Figure 1). Participants were given the Cancer Treatment Symptom Management Education: Preventing Dehydration booklet (developed by T.A. Ruegg) as part of an intervention toolkit during an in-person teaching session. This educational booklet describes information about the topics of chemotherapy, radiation therapy, dehydration, and oral mucositis. In addition to the booklet, the toolkit contained a cup, mug, pen, and flashlight to help encourage hydration and inspection of mucosa; a drinks diary for recording daily oral fluid intake; an oral assessment guide for referring to mucosal integrity (Eilers et al., 1988); and the Oral Mucositis Daily Questionnaire for daily completion related to mouth/throat soreness (Elting et al., 2008). After the in-person education session was conducted, participants received twice-weekly coaching telephone calls for the duration of their six- to seven-week chemotherapy and radiation therapy. Before implementing the intervention, the nurse and a backup person received a four-hour training course with the nurse researcher to ensure scientific rigor. 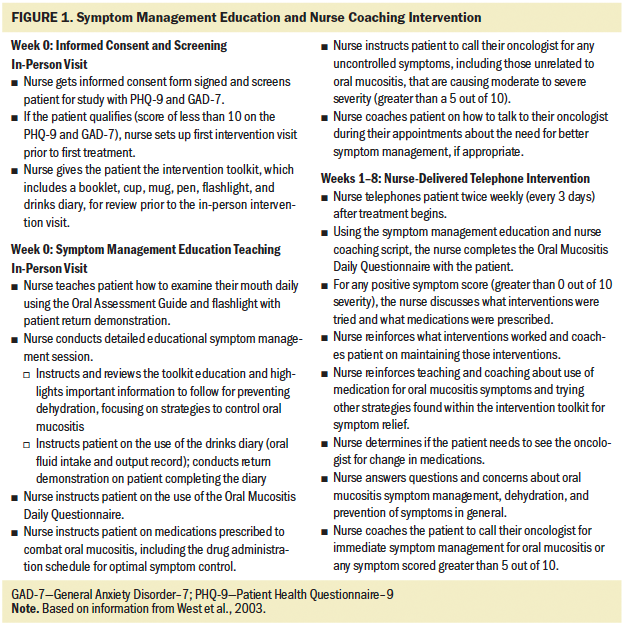
Participants, Setting, and Procedure
A two-phase, qualitatively driven, mixed-methods design (Morse & Neihaus, 2009) was conducted after institutional review board approval was obtained. During phase 1 of the study, participants received the tailored nurse-delivered coaching intervention throughout the chemotherapy and radiation therapy treatment, with data collected via quantitative instruments. Participants participated in phase 2 of the study three to four weeks after treatment concluded, undergoing a qualitative guided interview via telephone with the nurse researcher.
A nonprobability, purposive sampling technique was used to recruit participants. Inclusion criteria consisted of participants diagnosed with lung or head and neck cancer who were aged 18 years or older, able to read and write English or Spanish, were receiving combination chemotherapy and radiation therapy in the first-line treatment setting, and had an Eastern Cooperative Oncology Group performance status of 0–2 (ability to perform activities of daily living without the assistance of other people). Exclusion criteria consisted of being aged younger than 18 years, being blind or deaf, requiring assistance with activities of daily living, being clinically diagnosed with a cognitive impairment or with anxiety or depression (which could confound study results), and not owning a telephone. This study took place at ambulatory oncology clinical practice sites within the University of Miami Sylvester Comprehensive Cancer Center, an academic medical center in Florida. Eleven participants enrolled in the study, with 55% Hispanic ethnicity and male gender represented (see Table 1). 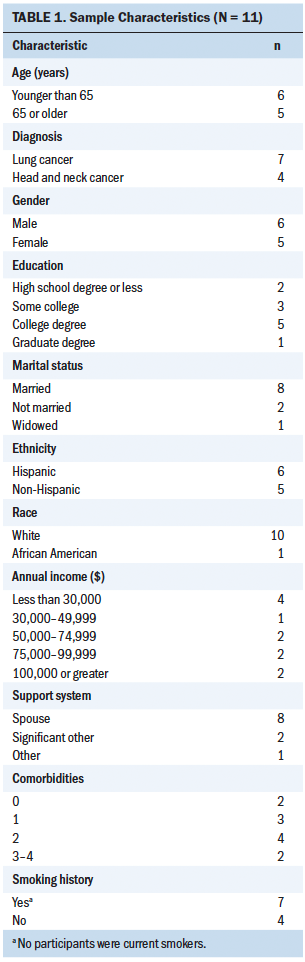
Although this feasibility study had no prospective randomized control group, data were included from a retrospective comparison control group. This comparison control group consisted of patients with the same inclusion and exclusion criteria as the intervention group except that the control group could not be prescreened for anxiety and depression in real time. To control for these criteria, patients with historical diagnoses of anxiety or depression noted in their medical records were excluded from participation in the comparison control group.
A qualitative guided interview and several validated quantitative instruments were used to implement this study. The qualitative guided interview was administered once; it was designed to determine if the nurse coaching intervention was feasible and sought to explore any unmet patient care needs that may have been encountered during treatment. Questions in the guided interview were designed to elicit participant responses specifically about the intervention and the associated sore mouth or sore throat symptom experience. Six questions were designed for the participant and focused on intervention feasibility, symptom experience, and symptom management, and one question was designed for the caregiver and based on the same topics.
A demographic questionnaire was administered at baseline. Anxiety and depression were measured at baseline using the General Anxiety Disorder–7 (GAD-7) (7 items, Cronbach’s alpha = 0.92) and the Patient Health Questionnaire–9 (PHQ-9) (9 items; Cronbach’s alpha = 0.89), respectively. GAD-7 scores range from 0 to 21, with higher scores indicating greater anxiety (Spitzer et al., 2006). PHQ-9 scores range from 0 to 27, with higher scores indicating greater depression (Kroenke et al., 2001). Scores of 10 or greater indicated anxiety or depression that was exclusionary for participation in the study, but no participants met the criteria.
Each health outcome variable was measured using quantitative survey tools at four time points: baseline, week 3, week 6, and end of treatment. Symptoms and symptom interference were measured with the MD Anderson Symptom Inventory (MDASI) for lung cancer (22 items; Cronbach’s alpha = 0.88 and 0.91) (Cleeland, 2007) and for head and neck cancer (28 items; Cronbach’s alpha = 0.88 and 0.92) (Rosenthal et al., 2007). MDASI scores range from 0 to 10 for each symptom item, with higher scores indicating greater symptom severity.
HRQOL was measured with the Functional Assessment of Cancer Therapy (FACT)–Lung (36 items; Cronbach’s alpha = 0.89 overall and 0.92 for total sum score; range of Cronbach’s alpha for all subscales = 0.82–0.88) (Cella et al., 1995) and FACT–Head and Neck (39 items; Cronbach’s alpha = 0.59–0.79 for all domains) (List et al., 1996). The FACT scales range from 0 to 144, with higher scores indicating higher HRQOL.
Symptom self-management was measured with the Partners in Health scale (12 items; Cronbach’s alpha > 0.8) (Peñarrieta-de Córdova et al., 2014; Petkov et al., 2010). Scores range from 0 to 96, with a stable or higher score from baseline indicating increased symptom self-management.
Perceived self-efficacy was measured with the Chronic Disease Self-Efficacy Scale (10 items; Cronbach’s alpha = 0.87–0.91) (Lorig et al., 1996). Scores range from 0 to 100, with a stable or higher score from baseline indicating increased perceived self-efficacy.
Oral mucositis symptom severity was measured daily (37–46 times) using the Oral Mucositis Daily Questionnaire that consists of eight questions (test-retest reliability coefficient = 0.7–0.9) (Elting et al., 2008). Unscheduled medical visits were measured against the retrospective comparison control group.
Mixed-Methods Analysis
The content analysis results of the qualitative core method of research formed the basis of the analysis. Quantitative supplemental survey results were analyzed by the Friedman test, and descriptive statistics were then integrated into the qualitative findings to add further information about the intervention’s effectiveness. The results narrative enabled a comprehensive research approach to answer the study’s specific aims (Morse & Niehaus, 2009).
Results
Qualitative Results
After the researcher concluded all interviews, a content analysis was performed using NVivo software, version 12. Three central categories, each with three related subcategories, emerged from the participants’ responses to the questions. Interpretive memos and field notes assisted the researcher in obtaining insight into the conceptual meanings of the participants’ text (Zhang & Wildemuth, 2010).
The three main categories were benefit of the nurse calls, intervention participation experience, and symptom experience (see Figure 2). Examples of each category, along with their subcategories coded from the participants, contributed to answering the primary and secondary aims of the study (see Figure 3). 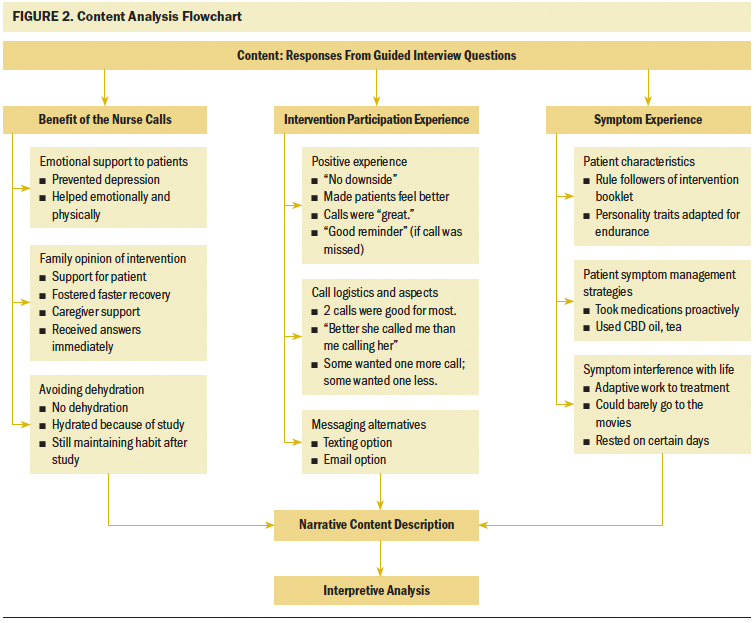
Benefit of the nurse calls (n = 5) consisted of participant views on nurse interventionist roles, including nurse educator and resource person, symptom monitor, and patient advocate. Other intervention benefits were found within the subcategories of avoiding dehydration, emotional support to patients, and family opinion of the intervention. Seven participants indicated that the nurse telephone calls were responsible, in some way, for them staying hydrated during their treatment. Participants (n = 5) reported that the nurse calls were instrumental in providing emotional support during treatment, and the calls prevented some (n = 2) from becoming depressed. The subcategory of family opinion of the intervention indicated that participants’ caregivers (n = 8) not only accepted the intervention, but also considered the calls an emotional benefit, particularly when receiving answers to questions immediately. 
Several responses to the guided interview questions formed the invention participation experience category. Subcategories of positive experience, evaluation logistics of the call, and messaging alternatives were the most relevant to the nurse interventionist calls. Responses exemplified critical information pertaining to the benefit and logistics of calls pertinent to meeting the elements of acceptability, demand, implementation, and practicality regarding intervention feasibility. Participants considered the nurse to be competent, liked someone “who knew about me,” and thought “it was good to have that interaction with someone about what was happening throughout the treatments.” The fact that the nurse was able to give help with “everything” was appreciated for most participants (n = 8).
The last category of symptom experience encompassed participants’ (n = 9) evaluation of treatment-related symptoms and the impact of managing symptoms. Patient characteristics, interference with life, and symptom management strategies were delineated from this main content category. Several participants (n = 6) found coping methods from symptom effects during their treatment phase; however, having a good support system at home was considered essential for enduring treatments. A slight majority of participants (n = 6) managed some symptoms with the help of the information they learned from the nurse before their treatment and using their home remedies, such as a special type of tea. Some participants (n = 3) expressed negative feelings about symptom interference with daily life. One participant tried to go the movies one time during his chemotherapy and radiation therapy but reported that it was difficult. Other participants (n = 3) made adjustments to their workplace or home environments as a way to minimize symptom interference.
Quantitative Results
The quantitative survey variables reinforced feasibility measures. The majority of the participants completed 80% of the intervention telephone calls, with demonstrated interaction with the nurse interventionist for at least five minutes per call. All participants demonstrated positive use of the intervention materials during the study period at least part of the time. Intervention practicality was established via the low attrition rate of 18%, with 100% of participants completing their cancer treatment.
The Friedman test statistic was nonsignificant for all secondary health outcome variables during the four time points; however, there were noted differences among the descriptive statistics. Median scores in overall symptom severity, HRQOL, perceived self-efficacy, and symptom self-management were reasonably close to baseline scores over time when analyzed descriptively, suggesting a possible intervention effect. These results are described as relatively low overall (see Tables 2 and 3). Oral mucositis symptom severity defined descriptively via daily scores were low, with more than half of participants having 0 as their median score. 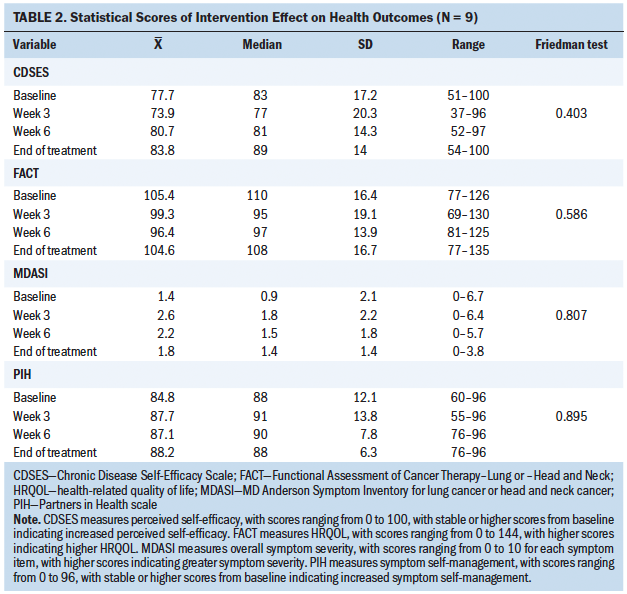
Analysis of participant drinks diaries and electronic health records showed that none of the participants had unscheduled medical visits to healthcare facilities for IV fluids, which would indicate dehydration. The participants’ verbal responses, coupled with the quantitative evidence involving the retrospective comparison control group analysis, suggested that the intervention had a positive effect in preventing dehydration. 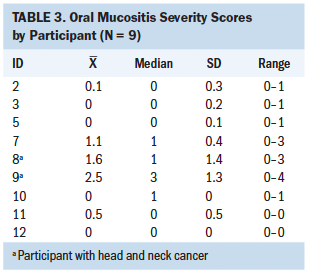
These findings suggest intervention feasibility. Nonparametric, descriptive statistical measures showed a potential effect of influencing the physiologic health outcomes of oral mucositis symptom severity and unscheduled medical visits. Mixed results were found among the health outcomes of overall symptom severity and HRQOL. The intervention had a variable effect on the behavioral outcomes of perceived self-efficacy and symptom self-management.
Discussion
Feasibility of Intervention
This study demonstrates intervention feasibility and that weekly telephone coaching affected the symptom severity of cancer treatment–related oral mucositis. These results are similar to those reported by studies using PRO-SELF program (Dodd & Miaskowski, 2000; Larson et al., 1998). The current study used a modified template of that program. Study participants reported that the intervention provided them with the benefit of cancer symptom management strategies and emotional support. These positive results from nurse coaching for self-management of symptoms are congruent with the outcomes found in other telephone coaching studies involving patients undergoing various cancer treatments (Coolbrandt et al., 2014, 2017; Howell et al., 2017; Kivelä et al., 2014; Suh & Lee, 2017; Thomas et al., 2012). However, most nurse-delivered telephone coaching studies only involve outcomes of pain and fatigue, with observed declines in symptom severity when compared to control groups (Fahey et al., 2008; Howell et al., 2017; Thomas et al., 2012).
The prevention of dehydration is a key factor in cancer disease sequelae and symptoms. Previous studies have not addressed this using a telephone intervention to prevent dehydration. The participant responses in the guided interview revealed that all were successful in remaining hydrated during treatment.
Intervention Logistics
The majority of the participants reported that receiving two weekly calls was acceptable; however, other participants offered alternatives. Once-weekly nurse coaching calls have successfully affected pain severity in patients with cancer (Fahey et al., 2008; Molassiotis et al., 2009; Thomas et al., 2012). Insight relating to why adjusted calls are acceptable (Kidd et al., 2009) include that high perceivers of control are less likely to rely on nursing interventions, and low perceivers of control are dependent on healthcare providers for their symptom management. In general, high perceivers of control take more responsibility for their self-care, whereas low perceivers of control tend to rely on others to guide them.
Alternatives to the telephone call, such as text messaging and email, were suggested by participants to increase communication with the nurse. Researchers have used email to enact automated symptom management modules to send symptom alerts to oncology provider teams for improved symptom control. In some trials, a nurse was still required to respond to the patient’s symptom severity alerts, indicating the need for personnel to control symptoms (Allen et al., 2008; Basch et al., 2016; Maguire et al., 2005; Mooney et al., 2017; Whitehead & Seaton, 2016).
Importance of Emotional Support
Emotional support emerged as a positive concept from the interviews from participants’ and caregivers’ perspectives. Systematic reviews showed that nurse-delivered telephone coaching improved emotional coping and the effect of symptoms (Howell et al., 2017). Future intervention research, including emotional components, is critical for success in improving long-term patient outcomes, as well as understanding how such interventions work to benefit patients.
Although automated systems have been found to help patients with cancer successfully manage their treatment-related symptoms at home, with decreases in symptom severity observed (Basch et al., 2016; Mooney et al., 2014; Sikorskii et al., 2007), automated systems do not provide the emotional support of a tailored nurse-delivered intervention. Participants felt that the nurse’s ability to listen to them provided valuable emotional support and verbal encouragement that friends sometimes did not offer. These elements are absent from studies using automated telephone coaching. A nurse was the preferred modality over technology-based symptom management monitoring systems in a large (N = 447) study by Kleiboer et al. (2010). However, different outcomes may be found in this study based on participant age and location; technology has progressed in usage and geographic area.
The caregivers of the participants with head and neck cancer reported that the support received from the nurse coaching intervention led to an early recovery for spouses. Most caregivers expressed their comfort from the nurse’s close monitoring. Caregiver inclusion in determining new intervention feasibility is paramount (Hopkinson et al., 2012; Kivelä et al., 2014; Schofield & Chambers, 2015).
Caregivers in this study expressed satisfaction with the education received throughout treatment to successfully care for the participant; not relying on external resources reduced their stress. Dyadic interventions provide emotional support for the caregiver, positively influence symptom management behaviors, and diminish caregiver emotional distress (Badger et al., 2013; Hopkinson et al., 2012; Northouse et al., 2010).
Intervention Effect on Health Outcomes
Of note, five participants (56%) experienced no oral mucositis symptoms. These findings were somewhat challenging to interpret because the research literature is slightly varied when reporting oral mucositis incidence rates. Some studies indicate a 30% incidence rate of low-grade mucosal esophagitis (Ozcelik et al., 2016; Yazbeck et al., 2013) and other systematic reviews exhibit a higher oral mucositis severity, leading to dehydration 30% of the time (Gong et al., 2016; Santana-Davila et al., 2015).
The rate of oral mucositis severity was higher in patients with head and neck cancer than in patients with lung cancer in this study, which is commensurate with the oral mucositis occurrence rate reported in the literature (Moslemi et al., 2016; Ray-Chaudhuri et al., 2013; Rodríguez-Caballero et al., 2012). However, scores were lower, differing from Trotti et al. (2003). Given the low occurrence rate of oral mucositis symptom severity in both participant cancer types as compared to the literature and reported participant engagement in the toolkit activities, the nurse coaching intervention may have reduced oral mucositis symptoms. Controlling for oral mucositis symptom severity, the authors hypothesized that overall symptom severity would be lessened. This hypothesis was not supported. Descriptive statistical results show that overall symptom severity was minimized because participants’ mean scores were low, suggesting that the intervention may have had some effect; however, the literature is scarce in reporting both outcomes synchronously (Chen et al., 2010).
The participant group median HRQOL scores involving patients with lung cancer were considered to be above average, indicating higher HRQOL; however, this is consistent with other studies (Auchter et al., 2001; Damm et al., 2013). Conversely, FACT–Lung results from participants with lung cancer were higher at all intervals than patients undergoing chemotherapy in a study by Kawahara et al. (2011). Klein et al. (2014) found that overall HRQOL scores declined during treatment in a systematic analysis of all treatments for head and neck cancer; however, scores returned to baseline within 12 months after treatment. Caregivers of the current participants with head and neck cancer reported that they believed the intervention made a difference in the participant’s recovery time, which, coupled with the moderate overall symptom severity observed, could be viewed as a potential intervention influence (if the sample size were comparative) when likened to the results of the systematic review by Klein et al. (2014).
Although the Friedman test demonstrated nonsignificance over time, individual descriptive findings showed that half of the participants seem inclined to favor supporting the theory of symptom self-management. As a group, when symptom severity increased, so did the group descriptive mean perceived self-efficacy scores (except at week 3). These findings are comparable to those of Coolbrandt et al. (2017).
The Chronic Disease Self-Efficacy Scale has been previously used in the population with cancer; however, the literature is scant. Perceived self-efficacy for symptom management instruments is also difficult to compare because of variance among the measurement scales (White et al., 2019). The participants in this study had higher overall Chronic Disease Self-Efficacy Scale scores at every measurement point when compared to patients with other diseases (Webel et al., 2013).
The Friedman test indicated that no intervention effect was found concerning symptom self-management. However, the group’s mean and median scores on the Partners in Health scale was relatively high overall throughout the study, beginning at baseline. Although the Partners in Health scale has been validated and shown to stabilize or increase if patients engage in self-management, the literature does not currently indicate standard ranges for any disease group. This study’s findings were higher than one study involving patients with cancer (Peñarrieta-de Córdova et al., 2014) but similar to involving another chronic disease (Cagnin et al., 2017), indicating that patients felt involved in their symptom self-management.
Unscheduled Medical Visits for Dehydration
The participants in this study had no unscheduled medical visits as compared to 40% of patients in the retrospective comparison control group, indicating a promising intervention effect. In analyses conducted with patients with head and neck cancer (Rodríguez-Caballero et al., 2012) and patients with lung cancer (Gong et al., 2016), about 15% of patients receiving chemotherapy and radiation therapy were hospitalized for treatment complications such as dehydration. Santana-Davila et al. (2015) found a dehydration rate of 21%–31% in 1,842 veterans receiving chemotherapy and radiation therapy for lung cancer at a U.S. Department of Veterans Affairs facility. Research has shown that these cancer types have uncontrolled symptoms, necessitating hydration (Elting et al., 2007; Terzo et al., 2017) and immediate care (Mayer et al., 2011; Ruegg, 2013). Because dehydration and uncontrolled symptoms often lead to costly unplanned admissions for these patients with cancer (Elting et al., 2007; Eskander et al., 2018; Peterman et al., 2001; Terzo et al., 2017), the nurse coaching intervention could be one promising way of helping patients stay hydrated and out of the emergency department.
Limitations
This study had several limitations. The first is related to the sample size, limiting ethnic diversity. Distance constraints between clinics and nurse interventionist time interfered with recruitment. Clinic appointment time changes affected communication with participants; however, the switch to using a cell phone midway through the study improved connectivity. Two male participants dropped out halfway through the study for treatment-induced pain. An attempt was made to control for response bias to decrease the threat to internal validity. Finally, the study was conducted at one institution, limiting generalizability.
Implications for Practice
The study results suggest that a tailored nurse-delivered telephone intervention is feasible and shows promise for affecting oral mucositis symptom severity and preventing dehydration for patients with lung or head and neck cancer and their caregivers. In addition, most participants reported other benefits of the intervention, such as patient education, resource connections, monitoring, advocacy, and overall support. Clinical practice implications may be significant in improving supportive cancer care. Patients with cancer could experience proactive symptom management while meeting physical needs. A more extensive study is essential to determine the overall benefit of health outcomes over time.
At this time of decreased health insurance reimbursement to facilities (Schneider & Hall, 2017), helping patients with cancer avoid unnecessary admissions for dehydration benefits health systems. Most importantly, nurse-delivered telephone coaching interventions can help patients with cancer live with the least amount of discomfort during cancer treatment while staying in the comfort of their own homes. 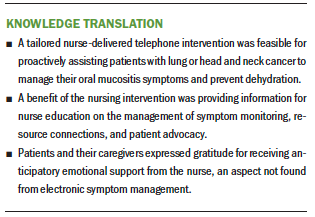
Conclusion
The tailored nurse coaching intervention in this descriptive pilot study is the first to prevent dehydration via telephone. The results indicate that there may be a potential effect on health outcomes. Emotional support via a nurse-delivered intervention was seen by the participants to be successful in helping them endure treatment, which was different from automated symptom management systems. These differences warrant future research in a larger, more controlled study.
The authors gratefully acknowledge Margaret Clayton, PhD, RN, Gilberto de lima Lopes, MD, Marjorie Pett, MStat, DSW, and Andrea Wallace, PhD, RN, for assistance with study conception, design, and analysis; Tejan DiWanji, MD, for participant recruitment; and A. Craig Lockhart, MD, MHS, for manuscript editing.
About the Author(s)
Tracy A. Ruegg, PhD, ANP-BC, AOCN®, is a medical oncology nurse practitioner at the University of Miami Sylvester Comprehensive Cancer Center in Florida; Janice M. Morse, PhD, RN, FAAN, is a distinguished professor and Barnes Presidential Chair in the College of Nursing at the University of Utah in Salt Lake City; and Raphael L. Yechieli, MD, is a radiation oncologist at the University of Miami Sylvester Comprehensive Cancer Center. This study was supported by American Cancer Society Doctoral Scholarships in Cancer Nursing (DSCN-16-067-01-SCN and DSCNR-18-073-03). All authors contributed to the conceptualization and design and manuscript preparation and provided analysis. Ruegg completed the data collection and provided statistical support. Ruegg can be reached at tar98@miami.edu, with copy to ONFEditor@ons.org. (Submitted August 2020. Accepted October 10, 2020.)




