Conservative Intervention Strategies for Adult Cancer-Related Lymphedema: A Systematic Review and Network Meta-Analysis
Problem Identification: The comparative effectiveness of available management options for cancer-related secondary lymphedema is unknown.
Literature Search: CINAHL®, Embase®, and MEDLINE® were searched for randomized trials comparing conservative treatment strategies.
Data Evaluation: A network meta-analysis was conducted for lymphedema volume, along with pairwise meta-analyses for remaining outcomes. Evidence certainty was assessed using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach.
Synthesis: Overall, 36 studies with a total of 1,651 participants were included. Compared to standard care, conservative treatments did not significantly reduce lymphedema volume. There was low to very low certainty evidence of benefit for several treatments on secondary outcomes.
Implications for Practice: There is insufficient evidence to suggest important differences between standard care and conservative treatment strategies for reducing lymphedema volume and improving lymphedema-related symptoms.
Supplemental material can be found at https://onf.ons.org/supplementary-material-conservative-intervention-strategies-adult-cancer-related-lymphedema
Jump to a section
Lymphedema is a localized swelling related to the collection of interstitial fluid resulting from improper lymphatic system drainage (Rockson, 2001). In addition to swelling, lymphedema is associated with a range of physical symptoms, including pain, heaviness, and tightness, as well as psychological symptoms, including distress, anxiety, and decreased quality of life (Fu et al., 2013). Primary lymphedema is attributable to an intrinsic fault in the lymphatic vessels, whereas secondary lymphedema is attributable to damaged lymphatic vessels or nodes, such as from surgery, radiation therapy, trauma, or infection (Shaitelman et al., 2015). Secondary lymphedema can be caused by lymphatic filariasis and cancer. Cancer-related lymphedema can be from breast, genitourinary, gynecologic, or head and neck cancers, as well as melanoma (Paskett et al., 2012).
Cancer-related lymphedema is a progressive chronic condition, with considerable burden on physical and psychosocial health, and it is associated with significant health system and out-of-pocket costs (Fu et al., 2013; Paskett et al., 2012; Shaitelman et al., 2015; Shih et al., 2009). It affects an estimated 5%–30% of cancer survivors, varying depending on the type of cancer, as well as other risk factors associated with cancer treatment (e.g., number of lymph nodes removed, number of sessions of radiation therapy), post-treatment care (e.g., infection prevention, surveillance), and patient characteristics (e.g., body mass index [BMI]) (Cormier et al., 2010; Jammallo et al., 2013; Shaitelman et al., 2015). The diagnosis of extremity lymphedema is related to the difference in the volume of the affected limb compared to the unaffected limb, or to the baseline limb volume prior to surgery. Lymphedema diagnosis is commonly based on the International Society of Lymphology criteria (Executive Committee, 2016). Stage 0 is considered latent or subclinical and is without obvious symptoms, stage I is a 6%–19% increase in volume (minimal lymphedema), stage II is a 20%–40% increase (moderate lymphedema), and stage III is a greater than 40% increase (severe lymphedema).
The current gold standard for lymphedema treatment is complex physical therapy, or complete decongestive therapy (CDT), which has two phases. The first is the treatment phase, which includes therapist-administered massage (manual lymphatic drainage [MLD]), compression (bandages, garments, and/or pumps), skin and nail care, and remedial exercise (specific exercises for the affected limb). The treatment typically lasts several weeks, with multiple sessions per week (Armer et al., 2013; Damstra & Halk, 2017). The second is the maintenance phase, which includes lifelong self-care to prevent recurrence and minimize the risk of complications, and usually consists of self-administered massage (simple lymphatic drainage), compression (bandages, garments, and/or pumps), skin and nail care, and remedial exercise. The complexity of self-care for lymphedema causes considerable patient and/or caregiver burden and cost, which may lead to low adherence and progression of lymphedema (Brown et al., 2014, 2015; Ridner et al., 2016; Shih et al., 2009). In addition, the treatment phase may need to be repeated if lymphedema symptoms are exacerbated (Damstra & Halk, 2017).
Several systematic reviews have been conducted on extremity lymphedema treatment strategies; however, these studies were overviews of reviews, were narrow in scope, or did not conduct a quantitative synthesis of findings (Douglass et al., 2016; Jeffs et al., 2018; Oremus et al., 2012; Singh et al., 2016; Stuiver et al., 2015). The current authors conducted a systematic review and meta-analysis of the most common conservative lymphedema treatment strategies to inform the Oncology Nursing Society clinical practice guideline on conservative intervention strategies for treating cancer-related extremity lymphedema among adult patients.
Methods
The current authors followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (see Appendix 1) and registered the protocol in PROSPERO (CRD42019119819).
Search Strategy
CINAHL®, Embase®, and MEDLINE® were searched from database inception to October 31, 2019, using a combination of controlled terms (Medical Subject Heading, Emtree) and keywords related to the concepts of lymphedema, the interventions considered (e.g., CDT, MLD, exercise, compression garments), and a randomized controlled trial (RCT) filter (McMaster HIRU). The authors searched for grey literature from two clinical trial registries: the National Institutes of Health (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform Search Portal (http://apps.who.int/trialsearch). Studies included in relevant systematic reviews were reviewed for any potentially eligible studies, as were reference lists of included articles. A health research librarian was consulted in developing the search strategy. Appendix 2 provides the full search strategy.
Study Selection
Reviewers (A.B.A., D.Z., K.N., L.L., M.S., P.K.G., R.L.M.) screened the titles and abstracts of identified citations and full texts of potentially eligible studies, independently and in duplicate. Disagreements were resolved by discussion or third-party adjudication, when necessary. An online systematic review software (Covidence) was used to facilitate literature screening. Two reviewers (K.N., L.L.) screened registered trials using an electronic spreadsheet.
Randomized trials concerning treatment of adult (aged 18 years or older) participants with cancer-related secondary lymphedema in the extremities published in English were included. Trials examining truncal, breast, and head and neck lymphedema were excluded because their diagnoses and treatment pathways are different from extremity lymphedema (Shaitelman et al., 2015; Smith & Lewin, 2010). Lymphedema was defined as volume change within three or more months after cancer treatment to exclude patients with temporary postoperative swelling (DiSipio et al., 2013). All author-reported volume thresholds for diagnosis were accepted. Studies with mixed populations—such as patients with primary and secondary lymphedema, patients at risk for and with diagnosed lymphedema, and patients with cancer- and noncancer-related lymphedema—that did not report the study population of interest separately were excluded. The secondary outcomes considered were lymphedema swelling and symptoms, return to work and activities of daily living, fatigue, function, quality of life, and pain (see Appendix, Table 3).
The authors included all conservative treatment strategies of at least two weeks in duration, including CDT, MLD, compression pumps, exercise (aerobic, resistance, weight training, yoga, water based), and standard care. Surgical treatments, pharmacologic treatments, laser therapy, kinesio tape, shock-wave therapy, electrical stimulation therapy, and aromatherapy were excluded, as were trials that compared different brands of the same medical device (e.g., compression bandages, garments, or pumps). Standard care was defined as self-management or the second stage of CDT (i.e., maintenance) and includes any combination of self-massage, compression bandages and/or garments, remedial exercises, and skin and nail care. Appendix, Table 4 presents details of intervention classifications.
Data Extraction
Reviewers (A.B.A., D.Z., K.N., L.L., M.J.Z., P.K.G., R.L.M.) independently extracted study data, including trial characteristics, intervention strategies, participant characteristics, and outcomes (e.g., measurement methods, effect estimates). Lymphedema volume was measured as volume calculated from circumference, water displacement, and bioimpedance spectroscopy. Single circumference measurements, or multiple circumference measures that were individually reported, were excluded, given the considerable measurement error associated with this method (Deltombe et al., 2007). Several studies reported multiple instruments measuring the same secondary outcome. To facilitate data extraction, for each secondary outcome (e.g., quality of life), the authors developed and systematically applied a hierarchy of the best instruments for each outcome and only extracted the outcome highest in the hierarchy. For studies that reported multiple time points, the longest follow-up time available was used. For crossover randomized trials, to exclude potential carryover effects, the authors included only the first phase of the trial. DigitizeIt, version 2.4.0, was used to extract data reported in graphs.
Mean and standard deviations (SDs) were extracted for all study outcomes. Methods described in the Cochrane Handbook for Systematic Reviews of Interventions were used to estimate the mean and SD when median, range, and sample size were reported and to impute SD if standard error was reported (Higgins et al., 2019). Change scores were preferentially analyzed from baseline to the end of follow-up to account for interpatient variability. For lymphedema volume, if studies reported baseline and end scores separately, the mean difference and associated SD were calculated using the mean correlation coefficient from studies that reported baseline, end, and change scores, as described in the Cochrane Handbook (Higgins et al., 2019). For secondary outcomes, only end scores were used because of insufficient data to calculate correlation coefficients.
Classification of Interventions
Reporting of interventions and cointerventions was inconsistent across studies. Consequently, in consultation with lymphedema experts (academicians and health professionals), the authors developed a system to classify the interventions reported in included studies. The authors considered the treatment phase of CDT to be comprised of four components: therapist-administered MLD, compression bandages and/or garments, skin and nail care, and remedial exercise. Remedial exercise was considered to be any variation of active, repetitive, nonresistive motion, as well as hand pumping and stretching of the affected limb, and breathing exercises. When authors classified interventions as CDT but did not report MLD or compression bandages and/or garments, the current authors classified only the reported intervention(s); however, if they did not report remedial exercise or skin and nail care, the current authors assumed there was a high likelihood that this was done and classified this as CDT. Compression pumps were classified as a separate intervention, even if study authors reported this as standard CDT. The maintenance phase of CDT was classified as standard care, including any combination of self-massage, compression garments, remedial exercise, and skin and nail care. Yoga exercise, tai chi–like exercise, and water-based (aqua lymphatic) exercise were combined as one intervention because they were deemed sufficiently similar by the lymphedema experts.
Risk-of-Bias Assessment
The Cochrane Collaboration risk-of-bias tool was used to evaluate individual RCTs, and the following domains were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other potential sources of bias (e.g., study funding, author conflicts of interest) (Higgins et al., 2011). The domains were rated as low risk of bias, unclear risk of bias, and high risk of bias.
The current authors considered that blinding of patients and personnel would not affect objective outcomes and so rated trials as having a low risk of bias for these outcomes, despite inadequate blinding. For subjective outcomes (e.g., quality of life, lymphedema swelling and symptoms), trials were rated as having a high risk of bias because of lack of blinding. A threshold of greater than 20% loss to follow-up was deemed to have a high risk of bias for incomplete outcome data. When authors did not report on loss to follow-up but all randomized participants were included in the analysis, trials were rated as having an unclear risk of bias for incomplete outcome data. Studies with prospectively registered or published protocols, where planned analyses matched reported analyses, were considered as having a low risk of bias for selective outcome reporting. Studies without registered or published protocols, or studies that were retrospectively registered, were considered as having an unclear risk of bias if all outcomes were reported in the methods and results sections and as having a high risk of bias when they were not. For other potential sources of bias, if studies reported industry funding and did not report the role of the funding source or had authors with industry affiliations, this was considered as having an unclear risk of bias. Studies with nonindustry funding, with or without industry in-kind donations of materials, were considered as having a low risk of bias. Studies where funding was not reported or where authors did not declare interests were also considered as having a low risk of bias for other biases.
Pairwise and Network Meta-Analyses
Because of variability in methods by which continuous outcomes were measured, the standardized mean difference (SMD) and the associated confidence intervals (CIs) were calculated. For interpreting effect size, an SMD of 0.2 was considered to be a small effect, an SMD of 0.5 a medium effect, and an SMD of 0.8 a large effect (Cohen, 2013). For pairwise meta-analyses (i.e., direct estimates) with at least two RCTs, the DerSimonian–Laird random-effects model was used. Heterogeneity between RCTs was assessed with the I2 statistic. For the network meta-analyses (NMAs), a frequentist random-effects under consistency model NMA was performed to calculate the direct and indirect treatment effects, assessing the comparative effectiveness of interventions (White, 2015; White et al., 2012). Incoherence (i.e., inconsistency in the model) was assessed by comparing direct estimates with indirect estimates using the node-splitting method, where incoherence is assessed locally by evaluating the coherence assumption in each closed loop of the network separately, as the difference between direct and indirect estimates for a specific comparison in the loop (Lu & Ades, 2006). Incoherence in the entire network was assessed using a design-by-treatment model (Higgins et al., 2012). No analyses were conducted to rank treatments because of limitations in using the approaches for low to very low quality estimates of effect (Mbuagbaw et al., 2017). Stata, version 15.1, was used for all statistical analyses. All comparisons were two tailed using a threshold of p ≤ 0.05.
Assessment of the Certainty in Evidence
For assessment of the certainty in evidence for the pairwise comparisons and NMAs, the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach was used (Brignardello-Petersen et al., 2018, 2019; Guyatt et al., 2008; Puhan et al., 2014). For direct estimates from RCTs, certainty in the effect estimates starts high and can be rated down because of risk of bias, inconsistency, indirectness, imprecision, or publication bias. The current authors could not test for publication bias because there were fewer than 10 studies for all comparisons. For indirect estimates, the current authors assessed first-order loops and used the lowest certainty rating from comparisons that informed the indirect estimate, and rated down further if evidence of intransitivity in the studies informing the direct comparisons was present (Chaimani et al., 2019). When determining the certainty of network estimates, the current authors used the highest of the direct or indirect estimate, and rated down for incoherence (statistically significant difference between direct and indirect effect estimates) and imprecision (wide CIs and/or sample size fewer than the optimal information size threshold of 400 observations) (Guyatt et al., 2011).
Results
A total of 3,186 unique titles and abstracts were identified for screening (see Figure 1). The current authors reviewed the full text of 152 articles, ultimately including 36 studies with 1,651 participants. Appendix, Table 5 provides the list of excluded articles, with reasons. 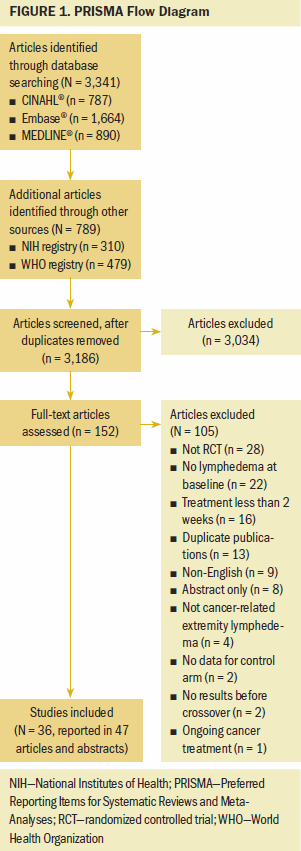
Study Characteristics
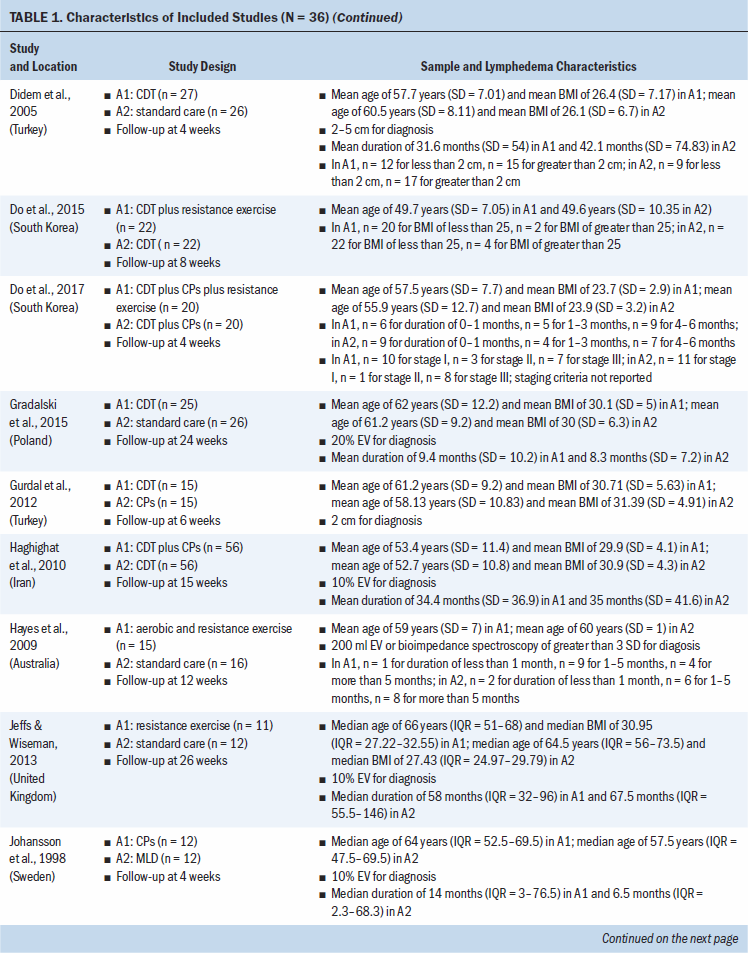
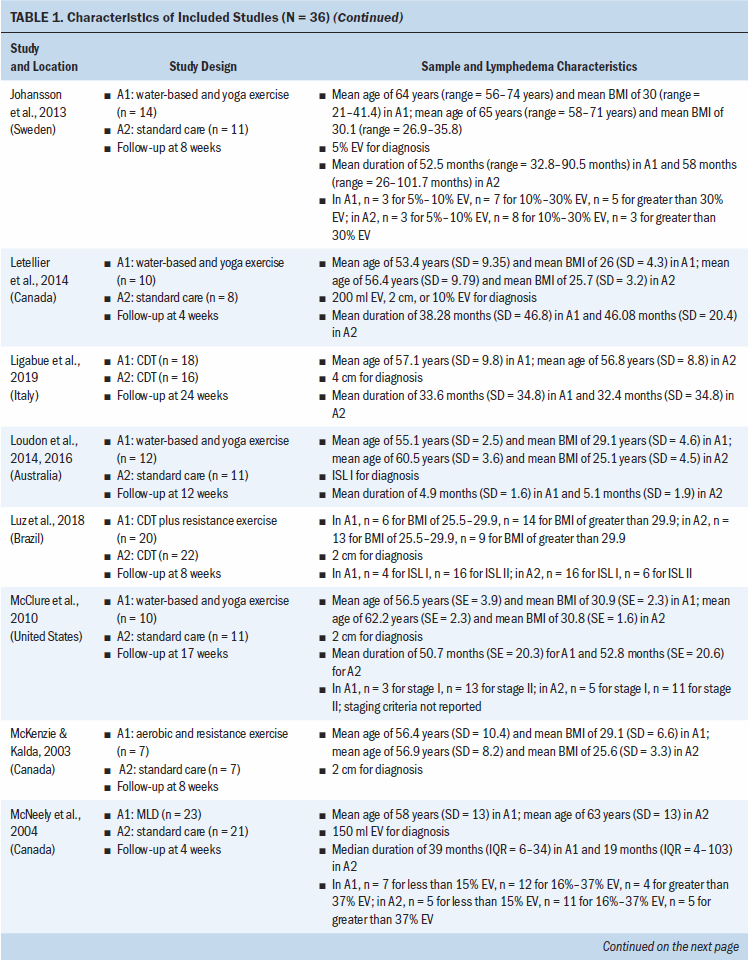
Study locations include Australia, Brazil, Canada, Denmark, Hungary, India, Iran, Italy, Poland, South Korea, Sweden, Turkey, the United Kingdom, and the United States (see Table 1). Interventions included CDT, MLD, resistance exercise, aerobic and resistance exercise, compression pumps, water-based and yoga- or tai chi–like exercise, and standard care. The length of treatment ranged from 2 to 52 weeks. The median follow-up time was eight weeks. Study sample sizes ranged from 11 participants (Wigg, 2009) to 139 participants (Schmitz et al., 2009, 2019). Most trials were small, with the majority having fewer than 50 participants (25 of 36, 69%). 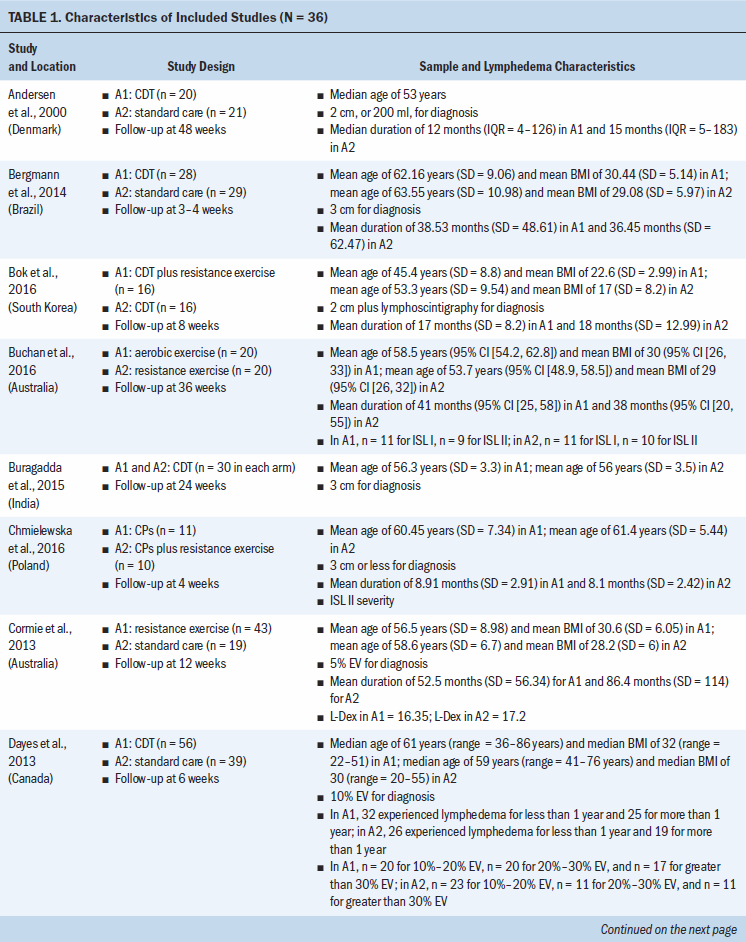
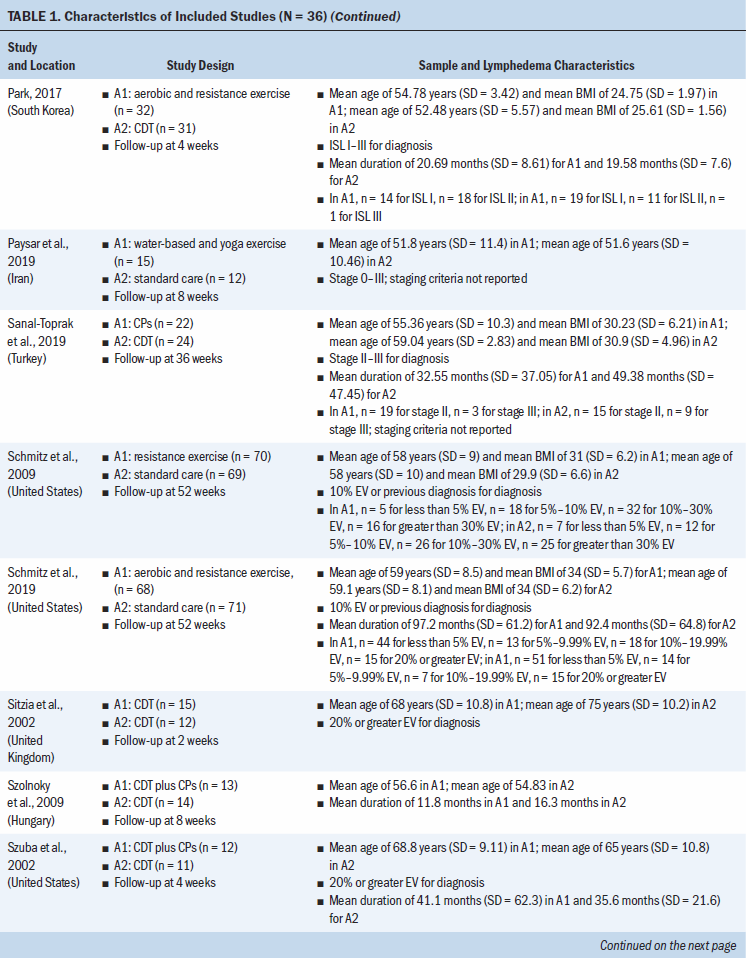
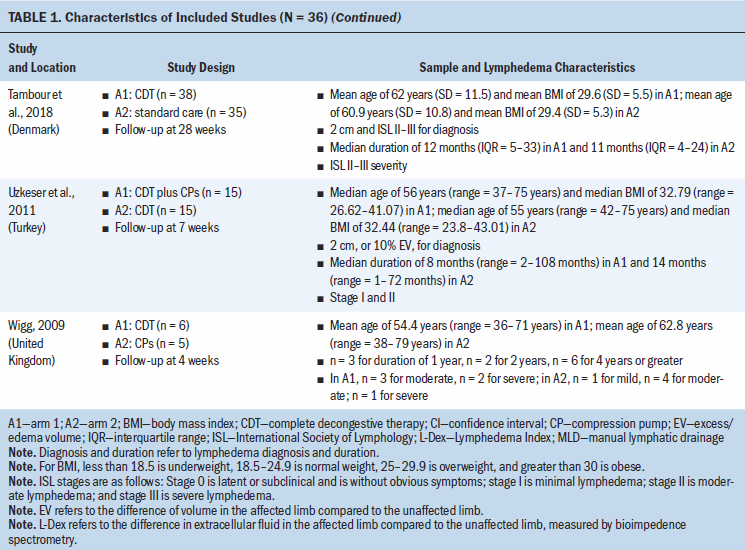
A total of 12 studies (33%) did not report a source of study funding; 18 (50%) reported funding from a university, hospital, and/or government; 4 (11%) reported no funding; and 2 (6%) reported university, hospital, and/or government funding and in-kind industry donation (see Appendix, Table 6). Among eligible trials, 19 (53%) had authors that reported no conflicts of interest, 16 (44%) did not report on author conflicts of interest, and 1 (3%) reported the lead author as holding a patent for a lymphedema treatment course.
Patient Characteristics
All trials included patients diagnosed with unilateral lymphedema, and diagnosis was based on volume difference between affected and unaffected limbs. Almost all trials (34 of 36, 94%) included participants with breast cancer–related lymphedema, with the exception of one trial that included participants who had gynecologic cancers (Do et al., 2017) and one trial that did not specify the patient population but reported that they had upper limb lymphedema (Wigg, 2009). All participants across trials were female. The mean age of participants ranged from 45.4 years (SD = 8.8) to 75 years (SD = 10.2). Among the 24 studies (67%) that reported BMI, mean BMI ranged from 22.6 (SD = 2.99) to 34 (SD = 6.2). Among the 28 studies (78%) that reported duration of lymphedema, mean duration ranged from 4.9 months (SD = 1.6) to 97.2 months (SD = 61.2).
Risk of Bias
All trials were at risk of bias for at least one domain (see Appendix, Table 7). Of the 36 trials, 18 (50%) were at low risk of bias for random sequence generation, and 17 (47%) were at low risk of bias for allocation concealment. Two studies were at high risk of bias for random sequence generation and allocation concealment (Luz et al., 2018; Uzkeser et al., 2011). Three studies (8%) were at high risk of bias because they had more than a 20% loss to follow-up. Three studies (8%) did not report whether any participants were lost to follow-up; as a result, they were considered to have an unclear risk of bias. Only seven studies (19%) had prospectively registered protocols. One study (3%) was at high risk of bias because the registered protocol methods did not match what was done in the final study, including changes in the secondary outcomes and a shorter time frame for analysis (Luz et al., 2018). One study was at an unclear risk of bias for other biases because it did not describe whether postrandomization exclusions because of ineligibility were made blinded to the treatment assignment (Luz et al., 2018).
Treatment Effect on Lymphedema Volume
Of 36 included studies, 27 studies involving 1,304 participants contributed to the network, with 10 nodes (see Figure 2). Reporting of lymphedema volume varied across studies, including mean difference of change from baseline, mean percent change from baseline, and baseline and end (i.e., follow-up) values. Studies not included in the network and reported narratively are presented in Appendix, Table 8. 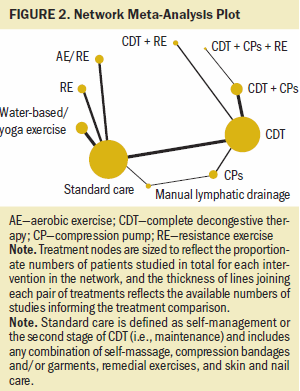
There was low to very low certainty evidence of meaningful change in lymphedema volume when comparing conservative lymphedema treatments (see Table 2; Appendix, Table 9; and Appendix, Figure 1). The evidence suggests that, compared to standard care, there is little to no difference in lymphedema volume changes from CDT (SMD = 0.07; 95% CI [–0.29, 0.43]), MLD (SMD = –0.33; 95% CI [–1.07, 0.41]), compression pumps (SMD = –0.08; 95% CI [–0.82, 0.66]), resistance exercise (SMD = 0.01; 95% CI [–0.48, 0.5]), and aerobic plus resistance exercise (SMD = 0.19; 95% CI [–0.34, 0.72]). In addition, there is little to no effect on lymphedema volume from water-based or yoga exercise (SMD = –0.29; 95% CI [–0.77, 0.19]), CDT plus resistance exercise (SMD = –0.26; 95% CI [–0.99, 0.47]), CDT plus compression pumps (SMD = –0.24; 95% CI [–0.84, 0.36]), and CDT plus compression pumps plus aerobic and resistance exercise (SMD = –0.13; 95% CI [–1.21, 0.96]), but the evidence is uncertain. 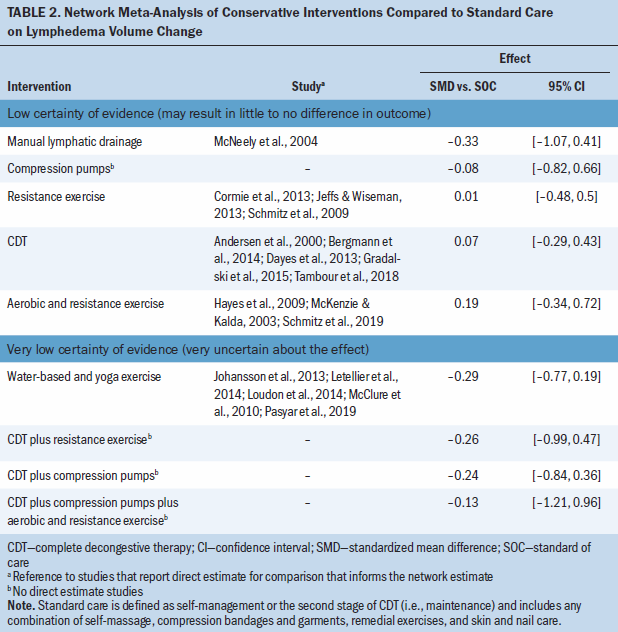
All comparisons were rated down for very serious imprecision. One direct comparison was rated down for inconsistency, and three were rated down for risk of bias. None of the comparisons were rated down for intransitivity. There was no incoherence identified for any of the individual comparisons in the network, and there was no statistical significance for local inconsistency (see Appendix, Table 10) or global inconsistency (p = 0.29).
Outcomes Informed by Pairwise Intervention Comparisons
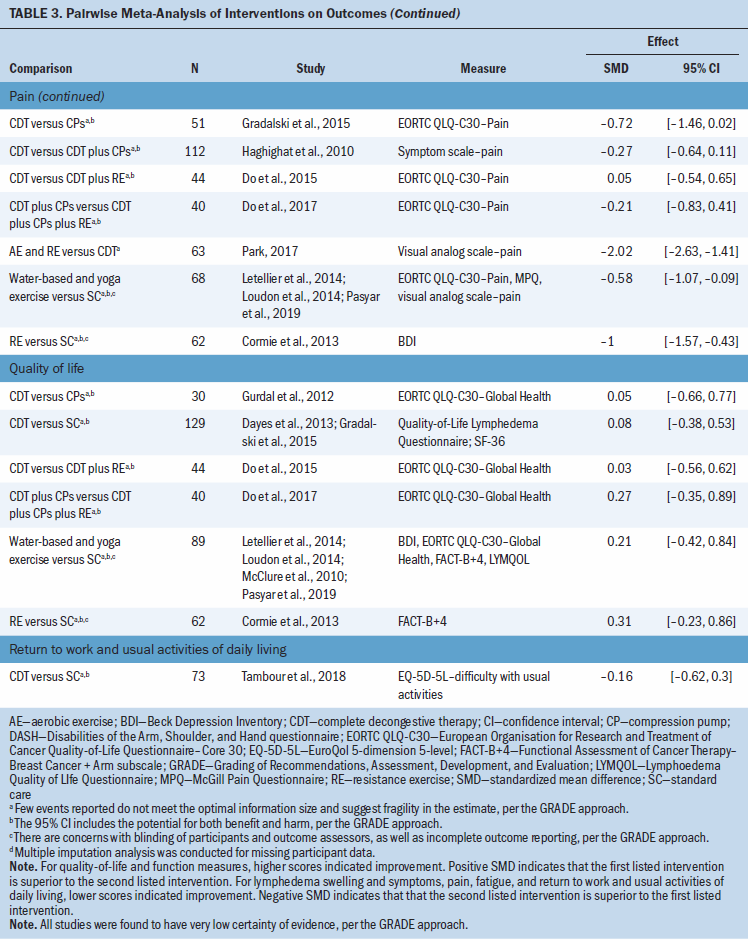
There was very low certainty evidence of a medium benefit for the aerobic and resistance exercise group compared to CDT for lymphedema swelling and symptoms (SMD = –0.38; 95% CI [–0.72, –0.05]) and a large benefit for function (SMD = 1.87; 95% CI [1.27, 2.46]) and for pain (SMD –2.02; 95% CI [–2.63, –1.41]), based on one study (N = 63 participants) (Park, 2017) (see Table 3). There was very low certainty evidence of a medium benefit for lymphedema swelling and symptoms for the CDT group compared to the CDT and compression pumps group (SMD = –0.4; 95% CI [–0.73, –0.06]), based on two studies (N = 139 participants) (Haghighat et al., 2010; Szolnoky et al., 2009). There was very low certainty evidence that resistance exercise compared to standard care has a large benefit for pain (SMD = –1; 95% CI [–1.57, –0.43]) and for function measures (SMD = 2.49; 95% CI [1.79, 3.19]), based on one trial (N = 62 participants) (Cormie et al., 2013), and also results in a medium benefit in lymphedema swelling and symptoms (SMD = –0.38; 95% CI [–0.72, –0.05]), based on one trial (N = 139) (Schmitz et al., 2019). Lastly, there was very low certainty evidence that water-based or yoga exercise has a medium benefit for pain compared to standard of care (SMD = –0.58; 95% CI [–1.07, –0.09]), based on three studies (N = 68 participants) (Letellier et al., 2014; Loudon et al., 2014; Pasyar et al., 2019). For several studies, it was not possible to include them in the meta-analysis, and their results are reported narratively (see Appendix, Table 11). 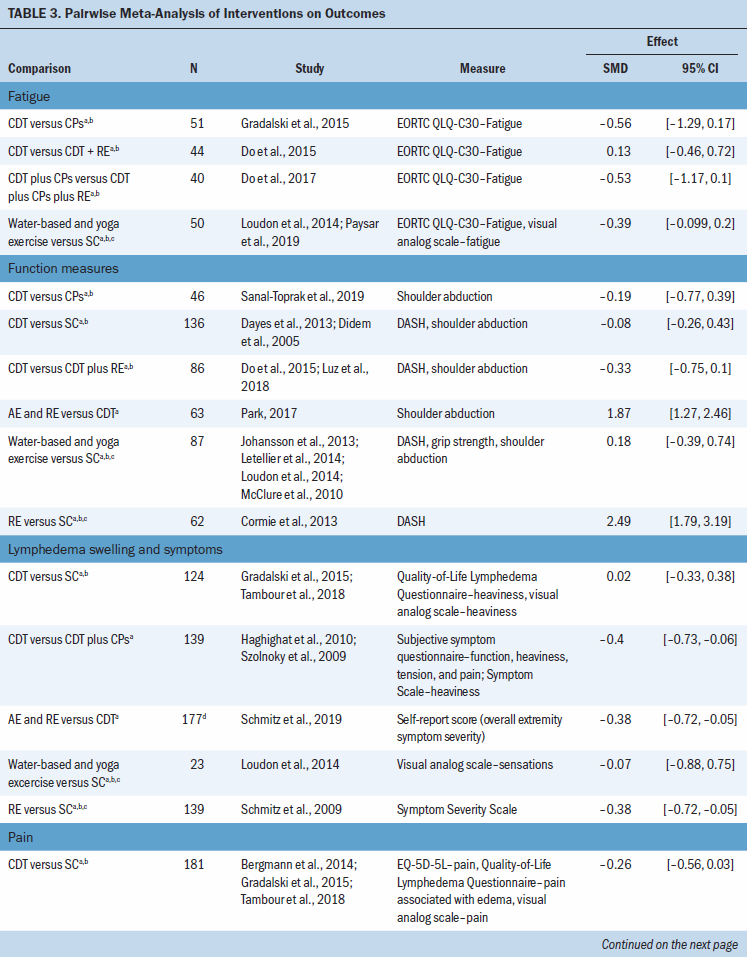
Adverse Events
Among the included studies, only nine studies reported about adverse events (see Appendix, Table 12). Of these, four studies stated that there were no adverse events, and three studies reported withdrawals from participants that included reasons potentially related to adverse events (e.g., cellulitis, reaction to compression bandage), although this was not explicitly stated by the study authors. The common adverse events reported included temporary rash, pain in the affected arm, skin reaction to bandaging, discomfort because of bandaging, lymphedema exacerbations, and infection/cellulitis.
Discussion
Statement of Findings
Lymphedema is a chronic and progressive condition that requires treatment and lifelong self-care. Among randomized trials on the different conservative strategies to treat cancer-related extremity lymphedema, there was no meaningful benefit of any of the interventions compared to standard care, based on low to very low certainty evidence. Several interventions had statistically significant, but small, effects on secondary outcomes; however, this was based on very low certainty evidence. There was limited evidence of adverse effects related to the treatments; however, this outcome was rarely reported among trials.
Importantly, there was variability across interventions and cointerventions, follow-up times, and outcome measures across trials. In addition, there is limited standardization of the available interventions, and authors may not adequately report this in trials. Both issues present challenges in generalizing the applicability of the findings. Also, there are no published trials on the minimally important difference in lymphedema volume reduction or on the additional outcomes for patients with lymphedema. Establishing minimally important differences would aid the interpretation of results (Sierla et al., 2018).
Context of This Review in Relation to Other Studies
A review by Sierla et al. (2018) examined measurement issues in lymphedema across 55 randomized trials and cohort studies, reporting four different instruments and 17 ways to present the outcomes. Several other reviews of primary and secondary lymphedema have discussed the challenges of drawing conclusions from the available data (Armer et al., 2013; Damstra et al., 2017; Finnane et al., 2015; Oremus et al., 2012; Sierla et al., 2018). Although the majority of previous reviews on lymphedema-related topics report data narratively, some authors have conducted pairwise meta-analyses. A review by Ezzo et al. (2015) conducted a meta-analysis of two studies that looked at the effect of MLD in addition to compression bandages (McNeely et al., 2004) and in addition to compression pumps (Johansson et al., 1998), finding a statistically significant effect of lymphatic drainage in addition to compression compared to compression alone. Three reviews pooled together trials of patients with or at risk of lymphedema and exercise interventions, with two finding that there was no statistically significant difference between exercise and nonexercise groups (Cheema et al., 2014; Paramanandam & Roberts, 2014) and one reporting decreased lymphedema volume (Rogan et al., 2016).
Strengths and Weaknesses of the Review
This review has several strengths. First, it is the largest conducted to date and features a search of multiple data sources, including grey literature. Second, it used well-defined criteria for study selection, as well as valid and comprehensive criteria to assess risk of bias. Third, it described the different interventions and cointerventions across studies, and the current authors consulted with clinical experts when making decisions about classifying and pooling interventions. Fourth, a random-effects model was used to account for heterogeneity between studies. Fifth, the GRADE approach was used to evaluate certainty in the estimates of effect.
This review also has a number of limitations. First, although it is a prevalent lifelong condition, lymphedema may be a secondary or tertiary description given to the study population and may be underreported in trials. As a result, the current authors’ search for and screening of the titles and abstracts may have failed to identify evidence that did not explicitly mention lymphedema as a health condition; however, the sensitivity of the search and screening in duplicate would have identified whether the treatment or management of lymphedema was the focus of the intervention or if any lymphedema-related outcomes were reported. Second, the authors did not look at component variations across studies (e.g., differences in types of massage techniques, pneumatic pumps, or compression garment types and wearing time), nor did they explore baseline adherence to self-care and how this could influence the effectiveness of the treatments. These questions were out of the scope of this review; however, they may provide meaningful context when choosing intervention modalities. Third, the authors made assumptions that all the data in the trials were normally distributed, but some trials reported that they had non-normally distributed data, and others provided ranges of outcome values that, combined with small sample sizes, suggested a non-normal distribution. This assumption influenced the inferences that could be made from the data, which may have resulted in the under- or overestimation of treatment effects. Fourth, SMD was used to calculate the estimates of effect, which can underestimate the incoherence in the network.
Unanswered Questions and Future Research
Patients, clinicians, researchers, and all relevant stakeholders should develop priority outcome sets to improve study design and reporting, which will facilitate between study comparisons and make future meta-analyses more feasible. In addition, such a core outcome set will give rise to the opportunity to include outcomes that matter to patients, other than volume of lymphedema. In addition, it would also be useful to develop a minimally important difference threshold.
Another potential approach to analyze the data, given the small sample sizes and variability across trials, as well as potential patient factors that influence relative effect of the treatments (e.g., lymphedema severity and duration), would be to conduct an individual participant data meta-analysis. Overall, further large randomized trials are needed to address the best strategies for treating cancer-related extremity lymphedema.
Implications for Nursing
CDT and continued self-care have been the gold standard for treating lymphedema for more than 20 years, but they are associated with considerable individual and health system burden and cost, in addition to issues of access to timely care (Daane et al., 1998; Kasseroller, 1998). Despite CDT being a component of the gold standard, the evidence for it is still limited. In addition, given the number of different devices available for lymphedema treatment (e.g., garments, compression pumps), it is important to critically evaluate therapies to identify ways to reduce burden and cost for patients, caregivers, and health systems. Of note, current lymphedema treatment guidelines are consensus based and/or outdated (Damstra et al., 2017; Harris et al., 2001), and it is imperative that high-quality guidelines supported by a systematic review of the evidence are developed.
Conclusion
There is low to very low certainty evidence that conservative treatment interventions may not meaningfully improve lymphedema volume compared to standard of care, and very low certainty evidence that some interventions may improve secondary outcomes associated with lymphedema. Generally conservative interventions are well tolerated; however, there was limited to no evidence on adverse effects. In addition, high-quality research is required to determine the efficacy and acceptability of conservative treatment strategies for treating extremity lymphedema.
About the Author(s)
Lyubov Lytvyn, MSc, is a doctoral candidate and Dena Zeraatkar, MSc, is a doctoral candidate, both in the Department of Health Research Methods, Evidence, and Impact at McMaster University in Hamilton, Ontario, Canada; Allison B. Anbari, PhD, RN, CLT, is an assistant research professor in the Sinclair School of Nursing at the University of Missouri in Columbia; Pamela K. Ginex, EdD, RN, OCN®, is a senior manager of evidence-based practice and inquiry at the Oncology Nursing Society in Pittsburgh, PA; Michael J. Zoratti, MSc, is a PhD candidate in the Department of Health Research Methods, Evidence, and Impact at McMaster University; Kacper Niburski, MA, is a medical student in the Department of Medicine at McGill University in Montreal, Quebec, Canada; Behnam Sadeghirad, PharmD, MPH, PhD, is an assistant professor in the Department of Health Research Methods, Evidence, and Impact and in the Department of Anesthesia, both at McMaster University; Madelin Siedler, MA, is a graduate teaching and research assistant in the Department of Physical Education and Exercise Science at the University of South Florida in Tampa; and Lehana Thabane, PhD, is a professor and Rebecca L. Morgan, PhD, MPH, is an assistant professor, both in the Department of Health Research Methods, Evidence, and Impact at McMaster University. Sadeghirad received a graduate student stipend from Mitacs Canada and received funding from PIPRA AG. Lytvyn, Anbari, Thabane, and Morgan contributed to the conceptualization and design. Lytvyn, Zeraatkar, Anbari, Ginex, Zoratti, Niburski, Siedler, and Morgan completed the data collection. Lytvyn, Niburski, Sadeghirad, Thabane, and Morgan provided statistical support. Lytvyn, Ginex, Thabane, and Morgan provided the analysis. Lytvyn, Zeraatkar, Anbari, Ginex, Zoratti, Niburski, Sadeghirad, Thabane, and Morgan contributed to the manuscript preparation. Lytvyn can be reached at lytvynlo@mcmaster.ca, with copy to ONFEditor@ons.org. (Submitted April 2020. Accepted May 10, 2020.)




