Interventions for Managing a Symptom Cluster of Pain, Fatigue, and Sleep Disturbances During Cancer Survivorship: A Systematic Review
Problem Identification: More than 40% of cancer survivors experience a symptom cluster of pain, fatigue, and sleep disturbances, which can greatly reduce quality of life.
Literature Search: A literature search was performed using CINAHL®, Google ScholarTM, PubMed®, PsycINFO®, and Web of Science. Articles included randomized controlled trials of interventions aimed at managing a symptom cluster of pain, fatigue, and sleep disturbances.
Data Evaluation: 11 studies were assessed for quality, risk of bias, cancer type, sample size, intervention type, duration, and setting. For an intervention to be considered effective, the reduction of symptom severity or distress had to be statistically significant.
Synthesis: Data from the 11 studies were summarized. Four interventions were found to be effective in managing the symptom cluster, but evidence on long-term effectiveness was insufficient.
Implications for Nursing: Oncology nurses can assess the patterns and characteristics of simultaneous symptoms experienced by cancer survivors and administer interventions to relieve symptom burden and improve quality of life.
Jump to a section
Early detection and advancements in cancer treatment have steadily increased survival rates among individuals with cancer. There are about 15.5 million cancer survivors worldwide, with approximately 1.7 million individuals entering cancer survivorship annually on average (National Cancer Institute, 2019; Siegel et al., 2019). In addition, cancer survivorship is projected to increase by 35% in the next decade (National Cancer Institute, 2019). Cancer survivorship encompasses the time from diagnosis through end of life and can include curative and maintenance treatments, secondary cancers, and remission (de Oliveira et al., 2018; Drury et al., 2017; Hebdon et al., 2015; Mullan, 1985).
During survivorship, cancer survivors may undergo curative or long-term maintenance therapies, develop secondary cancers, or enter remission, all of which have implications on physical, psychosocial, and financial issues related to well-being and quality of life (de Oliveira et al., 2018; Drury et al., 2017; Le Boutillier et al., 2019). In addition, cancer survivors often experience symptoms that can further affect functional status and quality of life (Deshields et al., 2014; Miaskowski et al., 2017; Mosher & DuHamel, 2012; Xiao, 2010; Xu et al., 2018). Previous studies have shown that cancer survivors often experience as many as 8–13 symptoms during survivorship (Barsevick, 2016; Deshields et al., 2014; Fan et al., 2007; Mosher & DuHamel, 2012). Symptoms can occur independently, but more frequently cancer survivors experience symptom clusters of two or more symptoms that coexist and may or may not have a common etiology, which can produce different outcomes than those expressed by a single symptom (Albusoul et al., 2017; Barsevick et al., 2006; Beck et al., 2005; Cheung et al., 2009; Dodd et al., 2001; Xiao, 2010). A symptom cluster of pain, fatigue, and sleep disturbances is the most common, occurring in more than 40% of cancer survivors (Kwekkeboom et al., 2009; Kwekkeboom, Tostrud, et al., 2018; Miaskowski et al., 2017; Mosher & DuHamel, 2012). However, cancer survivors also report individualized symptoms of pain, fatigue, and sleep disturbances about 75%–100% of the time during survivorship (Beck et al., 2005; Garrett, 2000; Kumar & Elavarasi, 2016; Smith & Saiki, 2015). Symptom clusters can create additional chronic conditions (e.g., depression, anxiety) that negatively affect prognosis and quality of life, ultimately leading to an increased use of the healthcare system and resources (Barsevick, 2016; Barsevick et al., 2006; Butt et al., 2008; Miaskowski et al., 2017; Palesh et al., 2007).
Reducing symptom burden in cancer survivors is crucial to improving quality of life and well-being (Albusoul et al., 2017; Le Boutillier et al., 2019; Miaskowski et al., 2017). Symptom management interventions can be developed by gaining an understanding of the physiologic and psychological mechanisms of symptoms, as well as preventing and managing symptoms or symptom clusters. Primarily, healthcare providers prescribe pharmacologic treatments, such as analgesics, psychostimulants, hematopoietic growth factors, or sedatives, for symptom management (Albusoul et al., 2017). However, pharmacologic treatments may inadvertently exacerbate other symptoms or produce new symptoms (e.g., using opioids to control pain may leave one feeling fatigued) (Kwekkeboom et al., 2009). Studies suggest that nonpharmacologic interventions, such as exercise, mindfulness-based strategies, and behavioral therapies, may positively affect social support, self-esteem, emotional distress, physical function, and overall quality of life for cancer survivors (Kwekkeboom et al., 2009; Miaskowski et al., 2017). These nonpharmacologic interventions can be an adjuvant to pharmacologic treatment regimens and enhance the survivorship experience.
A symptom cluster of pain, fatigue, and sleep disturbances is primarily triggered by cancer treatment and can persist throughout survivorship (Barsevick, 2016; Deshields et al., 2014; Dodd et al., 2001; Mosher & DuHamel, 2012; Mosher et al., 2017; Yennurajalingam et al., 2008). This symptom cluster can produce adverse side effects, such as interruption of functional ability, impaired role and social relationships, and exacerbation of underlying illnesses that ultimately decrease quality of life (Miaskowski et al., 2017). Therefore, interventions that target the symptom cluster of pain, fatigue, and sleep disturbances are needed. This systematic review aimed to assess the state of the science and effectiveness of pharmacologic- and nonpharmacologic-based randomized controlled trial (RCT) interventions that target and manage the symptom cluster of pain, fatigue, and sleep disturbances among cancer survivors.
Methods
Search Strategy and Data Sources
A comprehensive literature search was performed in CINAHL®, Google ScholarTM, PubMed®, PsycINFO®, and Web of Science according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2009). Studies published from 2001 to 2019 were included in the review, and search terms included pain, fatigue, sleep disturbance, and cancer. Filters were applied in Google Scholar to specify that all terms had to be present in the study’s title. A total of 1,025 articles were identified from the electronic database search, and 854 articles remained after duplicates were removed. After reviewing the article titles, abstracts, and methods, 33 full-text articles were screened for eligibility. Eleven studies met the inclusion criteria and were included in the review (see Figure 1). 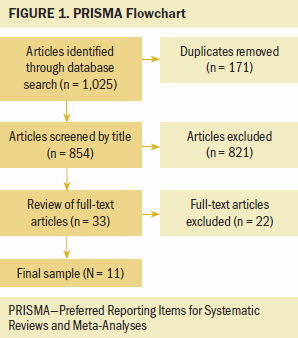
Inclusion and Exclusion Criteria
Eligibility criteria were developed to guide the selection of pertinent studies for this systematic review. Studies were included if they (a) involved cancer survivors aged 18 years or older, (b) used an RCT study design (nonpharmacologic or pharmacologic intervention), and (c) focused on, as well as individually measured and analyzed, symptoms of pain, fatigue, and sleep disturbances. Only articles published since 2001—when the construct of symptom clusters was introduced into cancer care (Xiao, 2010)—were included. Studies were excluded if they were pilot RCTs or written in languages other than English.
Data Extraction
Two independent authors used a two-step data extraction process for all studies that met the inclusion criteria. First, an author extracted information, including study design, sample characteristics, main aims, methods, interventions, measures, significant findings, and limitations, from each study. The second author then evaluated the first author’s data extraction process. The authors independently reevaluated each study and extracted data on the study settings, symptoms measured, and interventions used. Both authors assessed and identified relevant evidence regarding interventions for the symptom cluster of pain, fatigue, and sleep disturbances.
Quality Appraisal
The Quality Assessment Tool for Quantitative Studies, a quantitative scoring system, was used to evaluate the quality of the selected studies (Effective Public Health Practice Project, 1998). The Cochrane Collaboration’s tool for assessing risk of bias was used to assess the risk for bias in the selected studies (Higgins et al., 2019). The two authors assessed each study independently for quality assurance and risk of bias. If the authors disagreed, a third author was available for the additional assessment.
Coding
An intervention was considered effective if it demonstrated a statistically significant reduction in the symptom cluster’s level of severity or distress (p < 0.05). In addition, the feasibility and acceptability of the interventions were examined to determine whether cancer survivors and healthcare providers could incorporate the intervention into standard clinical care. Feasibility refers to whether the intervention was easily or conveniently applied in the clinical or home setting. Acceptability of the intervention was assessed by whether cancer survivors and healthcare providers maintained their involvement with the intervention during the study period. Feasibility and acceptability were based on attrition rate, with an attrition rate of less than 30% indicating that the intervention was acceptable and feasible. Interventions were considered partially effective if the intervention demonstrated feasibility or acceptability among cancer survivors or healthcare providers and the severity of or distress caused by one or two of the symptoms in the cluster decreased. Interventions that did not decrease symptom severity or distress were considered ineffective even if the intervention demonstrated feasibility and acceptability among survivors and healthcare providers.
Results
Study Characteristics
A total of 1,269 cancer survivors were included in the 11 studies reviewed (two studies reported on the same RCT). Five studies focused on breast cancer survivors; the six remaining studies focused on survivors of various cancers (e.g., gynecologic, prostate, lung, pancreatic, head and neck, renal, bladder). The following types of interventions were studied: exercise- or movement-based programs (n = 4), behavioral therapies or strategies (n = 3), pharmacologic therapy (n = 2), and stimulation-based therapies (n = 2). Interventions took place in the home setting (n = 2), the clinical setting (n = 6), or a combination of both (n = 2). Study characteristics are summarized in Table 1. 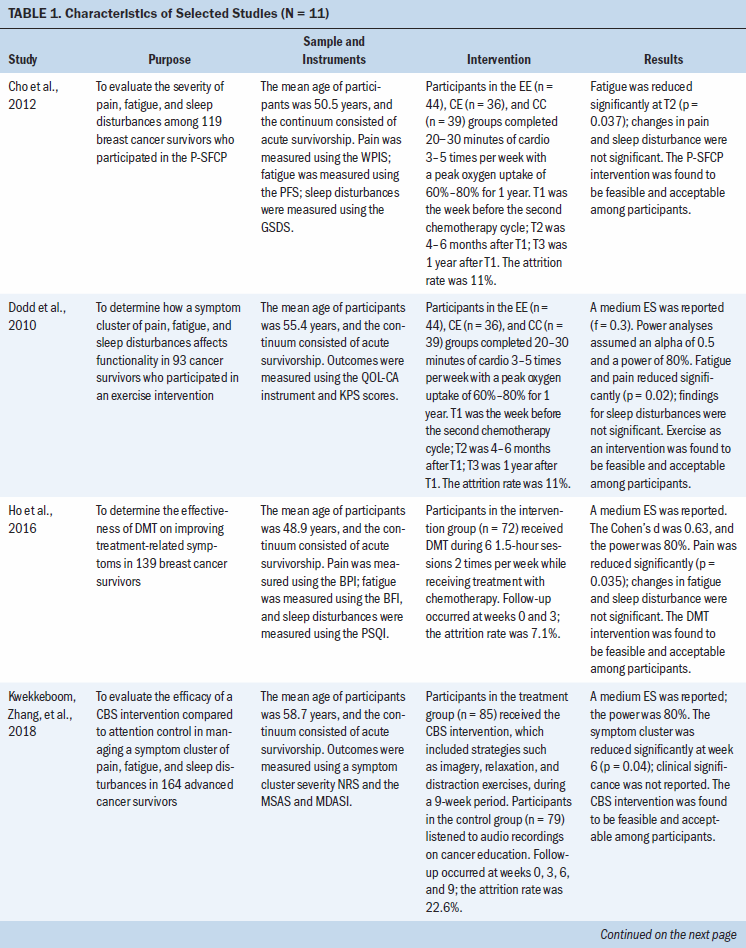
Intervention Types
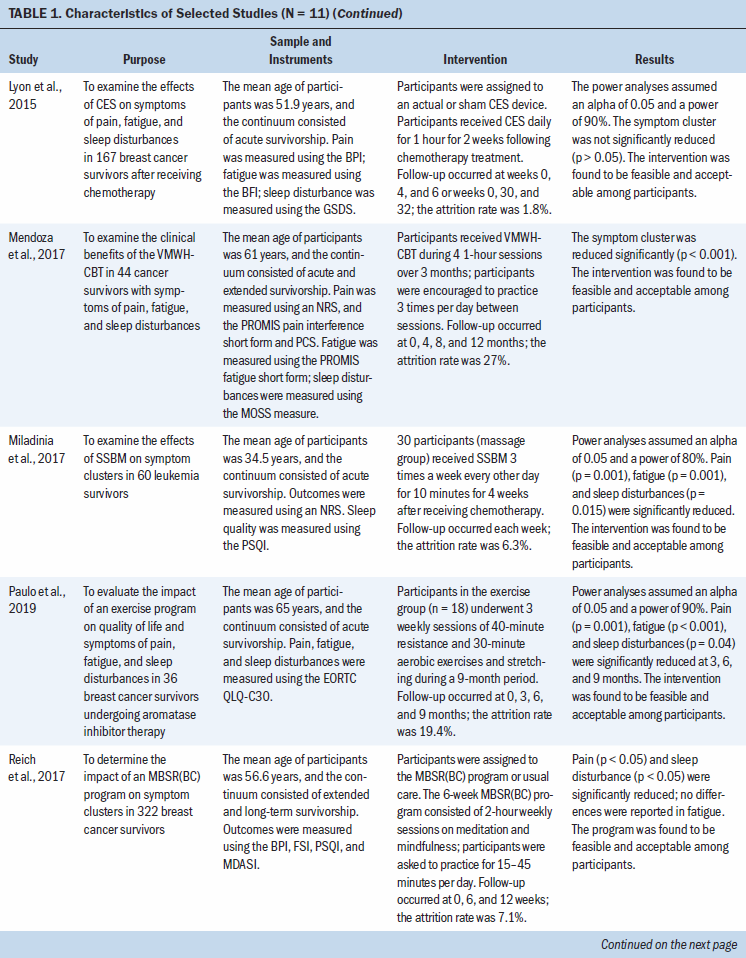
Exercise- or movement-based programs: Four studies used an exercise- or movement-based program as the intervention for managing symptom clusters. Cho et al. (2012) reported on symptom severity during the Pro-Self®: Fatigue Control Program (P-SFCP), which was a home-based program tailored to individual cancer survivors. Dodd et al. (2010) also evaluated a symptom cluster of pain, fatigue, and sleep disturbances and found that survivors reported that the severity of their fatigue (p = 0.02) and pain (p = 0.02) was reduced post-treatment. Although Cho et al. (2012) found that fatigue levels improved with exercise from baseline to four to six months (p = 0.037), exercise did not affect symptoms of pain or sleep disturbance during the 12-month study period. In addition, the P-SFCP intervention was shown to have a clinically significant reduction in symptoms of fatigue and pain but not sleep disturbances. In a 2016 study, Ho et al. determined the effectiveness of a twice-weekly intervention using dance movement therapy (DMT) throughout treatment with chemotherapy on improving treatment-related symptoms (pain, fatigue, and sleep disturbances) in breast cancer survivors. The DMT intervention was determined to be effective in managing pain (p = 0.035) but not fatigue or sleep disturbances; the decrease in reports of pain was believed to be clinically significant (Ho et al., 2016). In a study of older breast cancer survivors undergoing aromatase inhibitor therapy, Paulo et al. (2019) evaluated the impact of an exercise program on a symptom cluster of pain, fatigue, and sleep disturbances. The intervention was found to decrease symptoms of pain (p = 0.001), fatigue (p < 0.001), and sleep disturbances (p = 0.04) at the three-, six-, and nine-month time points compared to baseline. In addition, decreased reports of the entire symptom cluster suggest a clinically meaningful effect. Fidelity of the P-SFCP intervention was indicated (participants received weekly follow-up via telephone and at time points 2 and 3); however, fidelity of the DMT intervention or exercise program was not addressed (Ho et al., 2016; Paulo et al., 2019).
Behavioral therapy: Three studies used behavioral therapies or strategies alone or in conjunction with other interventions. First, Kwekkeboom, Zhang, et al. (2018) evaluated the efficacy of cognitive behavioral strategies, such as imagery, relaxation, and distraction exercises, to manage a symptom cluster of pain, fatigue, and sleep disturbances in advanced cancer survivors. Symptom distress was measured at baseline and at three, six, and nine weeks; a reduction in symptom distress from the symptom cluster was only reported at six weeks (p = 0.04). A clinically significant reduction in the symptom cluster was not reported. In a study of cancer survivors experiencing symptom clusters, Mendoza et al. (2017) investigated the clinical benefits of the Valencia model of waking hypnosis with cognitive behavioral therapy (VMWH-CBT) during four one-hour sessions over three months. The VMWH-CBT intervention effectively improved the entire symptom cluster (p < 0.001) and was found to be clinically significant (Mendoza et al., 2017). In two-hour weekly sessions over six weeks, Reich et al. (2017) evaluated the effects of a mindfulness-based stress reduction for breast cancer (MBSR[BC]) program on symptom clusters experienced by breast cancer survivors. The MBSR(BC) program was found to be effective in decreasing pain and sleep disturbances (p < 0.05), which was considered clinically significant, but it was not found to be effective in improving fatigue (Reich et al., 2017). Fidelity of the VMWH-CBT and MBSR(BC) interventions was reported. A mean fidelity score of 91% was reported for the intervention and control groups who received the VMWH-CBT intervention. The fidelity of the MBSR(BC) program was maintained through weekly follow-up using a checklist, which recorded the time and delivery of the intervention components.
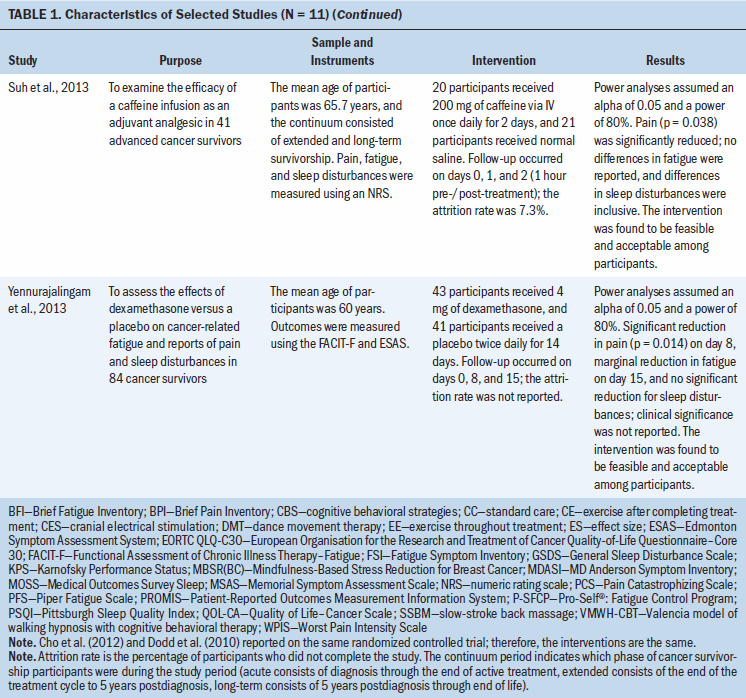
Pharmacologic therapy: Two studies aimed to examine the effects of pharmacologic interventions on symptoms experienced by cancer survivors. In a study by Suh et al. (2013), the efficacy of a caffeine infusion (200 mg for two consecutive days) as an adjuvant analgesic in advanced cancer survivors was assessed. The intervention was only effective in decreasing pain (p = 0.038) and did not affect fatigue or sleep disturbances. Intervention fidelity was not addressed (Suh et al., 2013). Similarly, Yennurajalingam et al. (2013) assessed the effects of dexamethasone (4 mg twice daily for 14 days) on symptom cluster severity; severity was measured on days 0, 8, and 15. Although the intervention was shown to be effective in reducing pain (p = 0.014) on day 8, no benefit was found on fatigue or sleep disturbances. Neither clinically significant differences nor fidelity were reported with the use of dexamethasone (Yennurajalingam et al., 2013).
Stimulation-based therapies: Two studies evaluated stimulation-based therapy, cranial microcurrent stimulation, and slow-stroke back massage (SSBM) to decrease symptom clusters in cancer survivors. In a study by Lyon et al. (2015), the effects of cranial electrical stimulation (CES) on breast cancer survivors’ symptoms (pain, fatigue, and sleep disturbances) during chemotherapy were examined. According to the results, CES did not improve the symptom cluster; clinically significant differences in the symptom cluster and fidelity of the intervention were not reported. Miladinia et al. (2017) evaluated the effects of a 10-minute SSBM intervention three times a week for four weeks on symptom clusters in leukemia survivors receiving chemotherapy. Although the study did not report clinically significant differences, the SSBM intervention demonstrated effectiveness in reducing the entire symptom cluster (p = 0.001 for pain and fatigue; p = 0.015 for sleep disturbances). Fidelity of the SSBM intervention was reported; massage procedures were assessed weekly for 10 participants and monthly for the remaining 20 participants (Miladinia et al., 2017).
Intervention Effectiveness
The interventions evaluated in the current systematic review demonstrated a range of effectiveness in managing either the entire symptom cluster or select symptoms. Intervention effectiveness was solely based on a statistically significant (p < 0.05) reduction in symptom distress or severity compared to the reported unstandardized clinically significant differences. The interventions in four studies were found to be effective (p < 0.05) in managing a symptom cluster of pain, fatigue, and sleep disturbances (Kwekkeboom, Zhang, et al., 2018; Mendoza et al., 2017; Miladina et al., 2017; Paulo et al., 2019). Two interventions managed two of the symptoms in the cluster (Dodd et al., 2010; Reich et al., 2017), four interventions managed one symptom in the cluster (Cho et al., 2012; Ho et al., 2016; Suh et al., 2013; Yennurajalingam et al., 2013), and one intervention was not effective at reducing any symptoms (Lyon et al., 2015). The types of interventions that were found to be effective varied, but they may share common biofeedback mechanisms, a technique to help an individual gain control and awareness over normal involuntary functions (e.g., pain, muscle tension, breathing). Differences were found among the interventions, including the type of intervention, the average length of time between the intervention and follow-up, and the heterogeneity of the cancer populations that were trialed.
Risk of Bias and Quality Assurance
Table 2 summarizes the selected studies’ overall risk of bias. The methodologic quality of the selected studies is reported in Table 3. 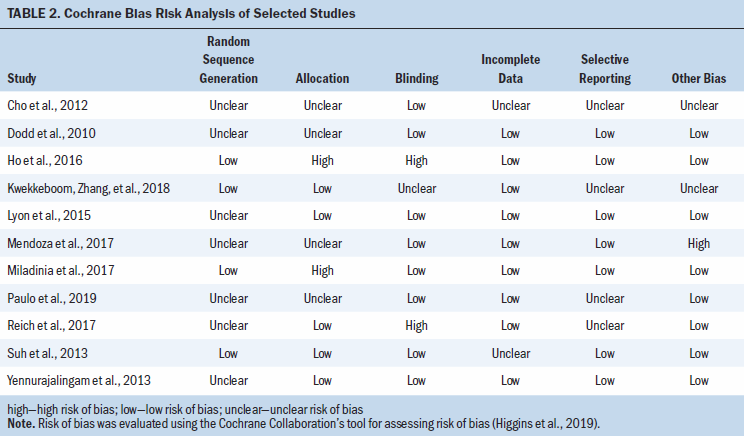
The most recently conducted RCTs were effective in managing the symptom cluster and presented with moderate- to high-quality assurance and low risk of bias compared to the noneffective intervention trials (Kwekkeboom, Zhang, et al., 2018; Mendoza et al., 2017; Miladinia et al., 2017; Paulo et al., 2019). Only four studies described the randomization process for the intervention or control groups, and four studies showed selection bias (Lyon et al., 2015; Reich et al., 2017; Suh et al., 2013; Yennurajalingam et al., 2013). In addition, four studies did not adequately describe the method for allocating the participants (Cho et al., 2012; Dodd et al., 2010; Mendoza et al., 2017; Paulo et al., 2019). Four studies did not specify whether the participants or researchers were blinded, and two studies did not blind the participants or researchers, which increased the potential risk of bias (Cho et al., 2012; Dodd et al., 2010; Kwekkeboom, Zhang, et al., 2018; Paulo et al., 2019; Reich et al., 2017). However, most of the studies used interventions that were not feasible in blinding the researchers to the control and intervention groups. 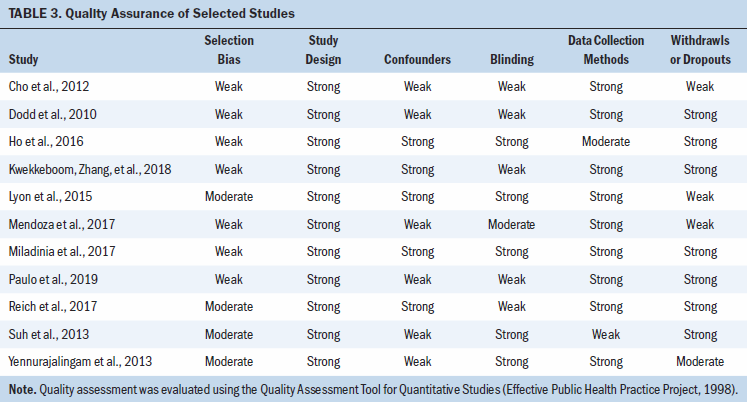
Discussion
To the authors’ knowledge, this is the first systematic review to examine the state of the science for RCTs of interventions aimed at managing a symptom cluster of pain, fatigue, and sleep disturbances. This review examined various types of interventions, including exercise- and movement-based therapy, behavioral therapy, cranial stimulation, pharmacologic therapy, MBSR, massage therapy, and hypnosis. All of the selected studies provided preliminary evidence of the intervention’s feasibility and acceptability among cancer survivors and healthcare providers. Intervention effectiveness for managing the individual symptoms or the entire symptom cluster varied. Four interventions reported a statistically significant improvement in the symptom cluster’s severity or distress (Kwekkeboom, Zhang, et al., 2018; Mendoza et al., 2017; Miladinia et al., 2017; Paulo et al., 2019), highlighting the need for additional interventions that can manage this symptom cluster in multiple cancer populations.
Four studies were found to be effective (p < 0.05) in managing the entire symptom cluster (Kwekkeboom, Zhang, et al., 2018; Mendoza et al., 2017; Miladinia et al., 2017; Paulo et al., 2019). In addition, Mendoza et al. (2017), Miladinia et al. (2017), and Paulo et al. (2019) reported clinically significant reductions in the symptom cluster, whereas Kwekkeboom, Zhang, et al. (2018) did not specify whether the reduction of symptom cluster distress at week six was clinically relevant. One significant benefit of the effective interventions was that the overall cancer populations studied were heterogeneous (e.g., breast and various cancers, leukemia, advanced cancer survivors), indicating that these interventions (exercise- and movement-based therapy, behavioral-based therapy, and massage therapy) can improve this symptom cluster in various cancer populations. Only four of the effective interventions reported whether their sample population participated in the intervention while receiving therapeutic treatment (Cho et al., 2012; Dodd et al., 2010; Lyon et al., 2015; Miladinia et al., 2017; Paulo et al., 2019).
The interventions in six studies were found to be partially effective in reducing symptoms of pain and fatigue (Cho et al., 2012; Dodd et al., 2010; Ho et al., 2016; Reich et al., 2017; Suh et al., 2013; Yennurajalingam et al., 2013). In the studies by Dodd et al. (2010) and Cho et al. (2012), the reduction of pain and fatigue was reported to be significant, and both studies reported clinically significant evidence. Reich et al. (2017) also reported that reductions in pain and sleep disturbances were clinically significant. Similarly, Ho et al. (2016) and Suh et al. (2013) reported that the reduction in pain was statistically and clinically significant. However, Yennurajalingam et al. (2013) did not report whether any reduction in pain was clinically significant. Only one intervention did not successfully reduce any symptoms in the cluster and resulted in no clinical significance (Lyon et al., 2015).
Feasibility and Acceptability
Similar strengths were found among the interventions in the selected studies related to preliminary feasibility, acceptability, and effectiveness among cancer survivors and healthcare providers. Feasibility and acceptability were not routinely reported in all of the studies, limiting the evidence. Acceptability was based on the active engagement of participants during the study period (attrition rate), suggesting that cancer survivors and healthcare providers maintained their involvement because of the feasibility of implementing (convenience of integrating) the interventions in both clinical and home settings. However, intervention effectiveness was not dependent on the setting (home or clinical). For example, interventions using imagery, relaxation, distraction, or massage therapy could be provided to cancer survivors during their clinical appointments or while they are receiving treatment, and exercise therapy could be provided as an outpatient rehabilitation resource. These effective or partially effective interventions could be maintained as standard medical care at comprehensive cancer centers, but larger RCTs are needed to demonstrate the stability of efficacy, feasibility, and acceptability over time. Providing readily available interventions to cancer survivors that can be maintained in daily life can help to ensure that survivors receive the full therapeutic benefits of the intervention. In addition, the effective interventions in this review had diverse content, conditions, sample characteristics, and durations, which may explain differences in outcomes on symptom cluster management.
Intervention Limitations
Five of the studies in this review were conducted with breast cancer survivors, presenting relative selection bias. The majority of the studies had a homogeneous sample (e.g., race and ethnic background, gender, income), limiting generalizability to other types of cancer. Technical study faults (e.g., lack of reporting randomization methods) were also found, which suggests that future RCTs should randomize participants to adequately ensure rigor and reproducibility. Only four of the RCTs described the randomization process used. Poor randomization can increase the risk for selection bias, which may contribute to false positive results and indicate that a statistically significant reduction in the symptom cluster may not be valid.
In addition, only four of the RCTs addressed the fidelity of the interventions used. Because adherence to the interventions was not reported, the ability to effectively reproduce the interventions is limited. Instruments used to measure the symptom cluster were inconsistent, which may complicate generalizability among interventions and the ability to determine statistically significant reductions in the symptom cluster. The feasibility of the interventions was also not adequately addressed, making it difficult to reliably assess whether the interventions maintained feasibility and acceptability among cancer survivors and healthcare providers over time, as well as a lack of reporting on participant attrition. Not all studies reported on clinically significant differences in the symptom cluster; a reduction in a symptom may have clinical significance but not achieve statistical significance. The components of the intervention protocol(s) were not well described, limiting the ability to validate findings.
The four effective interventions (Kwekkeboom, Zhang, et al., 2018; Mendoza et al., 2017; Miladina et al., 2017; Paulo et al., 2019) were similar in the number of times that participants engaged with the intervention (a few times a week to a few times daily). Follow-up time points varied from 2 days to 12 months, limiting the ability to assess the effectiveness of the intervention on managing the symptom cluster over time. Assessments of the longitudinal effects of the interventions on the symptom cluster were inconsistent, which indicates that the interventions may not maintain effectiveness over time. Collectively, these limitations suggest that future RCTs would benefit from including larger sample sizes across diverse geographic locations and multiple cancer populations to confirm the effectiveness of these preliminary findings. Longitudinal data can provide insight into the stability of these interventions over time.
Study Limitations
This systematic review only included studies written in English from five databases, which may have provided incomplete information and created publication bias. In addition, the interventions in this review were only considered to be effective based on a statistically significant reduction (p < 0.05) in the symptom cluster; it is possible that a statistically significant reduction does not indicate a clinical reduction in the severity of or distress caused by the symptom cluster. Symptoms are often complex and multidimensional, which highlights the importance of developing standardized measures and consistently using cut points in reporting data. Well-developed RCTs with larger sample sizes and long-term follow-up time points are needed to confirm the effectiveness of interventions for the management of this symptom cluster among cancer survivors.
Implications for Nursing
This systematic review provides preliminary evidence on interventions for managing a symptom cluster of pain, fatigue, and sleep disturbances among cancer survivors throughout survivorship. Additional research is needed to explore the benefits of exercise- or movement-based, behavioral-based, and massage-based interventions in larger cohorts to confirm their effectiveness and stability over time (Xiao, 2010). The clinical implications are unclear primarily because the literature is not well developed, making it difficult to provide reliable and clear recommendations (Fleishman, 2004; Miaskowski et al., 2017; Xiao, 2010). Nevertheless, oncology nurses need to continue to advocate for cancer survivors and assess their symptom characteristics (e.g., frequency, patterns, severity, distress) to provide insight that can be used to facilitate the development of interventions targeting symptom clusters of pain, fatigue, and sleep disturbances in this population.
Nurse educators and scientists can explore common biofeedback mechanisms that may link the interventions that have been shown to be effective. It would also be beneficial for nurse scientists to investigate the common biofeedback mechanisms in future studies to improve the overall efficacy of the interventions. Nurse scientists who are researching symptom clusters should provide clarity and consistency on specific instruments used to measure symptom clusters and describe the randomization process adequately to aid in the discovery of a common symptom cluster etiology (Chen et al., 2011). In addition, the development of a broad array of interventions delivered across multisite facilities that include diverse cancer populations should be considered a priority to determine the potential for outcome differences, as well as whether cancer survivors and healthcare providers adhere to symptom management interventions. For a sustained impact on the well-being and quality of life of cancer survivors, interventions need to be thoroughly tested using longitudinal study designs to ensure reliability and stability in outcomes, primarily because symptom clusters may persist or evolve during cancer survivorship (Fleishman, 2004; Jhamb et al., 2019; Miaskowski et al., 2017). 
Conclusion
To the authors’ knowledge, this was the first systematic review to provide an overview of intervention research targeting a symptom cluster of pain, fatigue, and sleep disturbances among cancer survivors. Three types of interventions (massage therapy, behavioral-based therapy, and exercise- or movement-based therapy) were found to be effective in alleviating symptoms of the entire cluster. However, effectiveness of the interventions in managing this symptom cluster varied, and the lengths of the interventions, the feasibility and acceptability of the interventions, the instruments used to measure symptoms, and the clinical evidence were inconsistent across all studies reviewed. Many interventions are aimed at managing this common symptom cluster during cancer survivorship. Future research needs to focus on developing effective interventions aimed at reducing the severity of this symptom cluster to significantly improve the quality of life and well-being of cancer survivors.
About the Author(s)
Sameena F. Sheikh-Wu, RN-BC, BSN, BA, is a PhD student and research assistant, Charles A. Downs, PhD, ANCP-BC, FAAN, is an associate professor, and Debbie Anglade, PhD, RN, MSN, LHRM, CPHQ, CCM, is an assistant professor, all in the School of Nursing and Health Studies at the University of Miami in Florida. No financial relationships to disclose. Sheikh-Wu and Downs contributed to the conceptualization and design and provided the analysis. Sheikh-Wu completed the data collection and provided statistical support. All authors contributed to the manuscript preparation. Sheikh-Wu can be reached at sfs23@miami.edu, with copy to ONFEditor@ons.org. (Submitted December 2019. Accepted February 20, 2020.)




