Ethical Challenges Encountered by Clinical Trials Nurses: A Grounded Theory Study
Objectives: To investigate the ethical challenges experienced by oncology clinical trials nurses (OCTNs) during the management of CTs and to examine how they resolve those conflicts.
Sample & Setting: 12 licensed RNs who had been practicing as full- or part-time OCTNs for a minimum of two years at various academic medical centers in the United States.
Methods & Variables: Classical grounded theory (CGT), an inductive methodology used to explore a social process in which little is known and to develop a theory grounded in the data, was used, in addition to CGT data analysis strategies.
Results: CGT data analysis revealed the OCTNs’ main concern (implementing an undefined job) and the way in which the OCTNs resolve this concern through the process of figuring it out. Figuring it out consists of learning as they go, utilizing their assets, standing their ground, and managing hope.
Implications for Nursing: Although some nursing research provides examples of ethical challenges OCTNs might encounter in practice, there is little information regarding how nurses manage those encounters. A theoretical understanding of the OCTNs’ experiences managing ethical challenges fills a gap in the nursing literature and provides a framework for how OCTNs manage and respond to challenges in professional practice.
Jump to a section
The American Nurses Association (ANA) and the International Association of Clinical Research Nurses (IACRN) in 2016 published Clinical Research Nursing: Scope and Standards of Practice, which describes the professional role and obligations of the clinical trials nurse (CTN). That same year, the Oncology Nursing Society (ONS, 2016) published 2016 Oncology Clinical Trials Nurse Competencies, standardizing the practice for oncology CTNs (OCTNs) by identifying the knowledge and behaviors of those in this role.
Nurses can assume responsibility for a CT and patients taking part in the CT by recruiting patients, overseeing the informed consent process, managing data, and ensuring regulatory compliance (Offenhartz et al., 2008). Various publications (Kunhunny & Salmon, 2017; Ness & Royce, 2017; Purdom et al., 2017) have provided further explanation of the roles and responsibilities of the CTN and the OCTN in practice. Nursing involvement in CTs is essential to ensure that the ethical responsibility to the patient is met. CTNs constantly balance the clinical care of the patient taking part in the CT and the obligations to the research study by protecting patients while ensuring that quality data are collected.
Much of the literature describes the role of the CTN and the domains of the CTN specialty (ANA & IACRN, 2016; Bevans et al., 2011; Castro et al., 2011; Di Giulio et al., 1996; Hill & MacArthur, 2006; Mori et al., 2007; Offenhartz et al., 2008). Other research has identified the ethical challenges that could arise in protocol compliance and documentation, as well as in the management, recruitment, and retention of patients (Barrett, 2002; Cantini & Ells, 2007; Chamorro & Appelbaum, 1988; Cisar & Bell, 1995; Di Giulio et al., 1996; McEvoy et al., 1991; Ocker & Pawlik Plank, 2000).
Cox and Avis (1996) noted that ethical challenges can develop during CTs that threaten CTNs’ and OCTNs’ belief in autonomy, justice, informed consent, disclosure of information about treatment, and patient decisions concerning CT participation. A lack of role clarity or conflicted allegiances can leave CTNs feeling caught between obligations to the patient and obligations to the CT (Larkin et al., 2019; Mueller, 2001; Mueller & Mamo, 2002; Wilkes & Beale, 2005). CTNs may find themselves “in a morally difficult position when having to make a decision about whether to act on perceptions of patients’ best interests or to follow the study protocol” (Oberle & Allen, 2006, p. 183). Although Larkin et al. (2019) acknowledge that support, research, and education should be directed to nurses who experience distress related to managing ethical challenges, there is little information on how CTNs can manage ethical challenges.
No research has been identified that used a qualitative approach to address the OCTN perspective concerning ethical challenges experienced during the management of CTs. In addition, there are no existing theories addressing the perceptions of OCTNs managing ethical challenges encountered during CTs. The current study used classical grounded theory (CGT) (Glaser, 1978, 1998) to examine ethical challenges experienced by OCTNs by exploring the following research question: What are the perceptions of the OCTN regarding the ethical challenges experienced in professional practice?
Methods
Participants and Setting
A study website was created to recruit participants and collect data using an online synchronous typed format in which the participant and the researcher (S.G.F.) communicated in real time by typing questions and responses. The website was password protected, and data were collected within each participant’s password-protected designated webpage. The website provided an additional level of privacy for participants and allowed the researcher to engage participants from a broader geographic area.
The study used purposive and snowball sampling to recruit participants. The researcher discussed the study with colleagues during events such as an international conference and pharmaceutical nursing advisory boards. The 12 participants were licensed RNs who had been practicing as full- or part-time OCTNs for a minimum of two years (see Table 1). Participants worked at various academic medical centers in the United States in an OCTN role combining the direct patient care role and the coordinator role. All could speak and write in English, had access to a computer, and possessed the computer skills necessary to participate in online data collection. 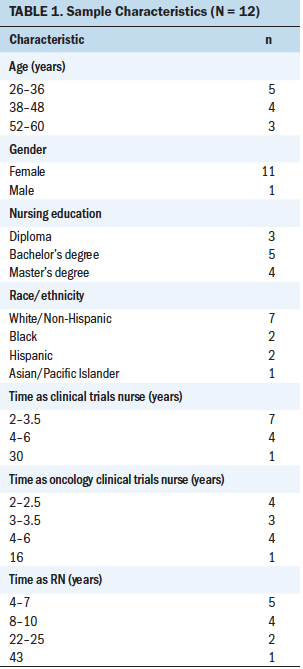
Methodologic Approach
The current study used CGT, a rigorous, inductive research approach that systematically collects data about social processes, identifies concepts, and finds relationships among those concepts (Glaser, 1978, 1998). CGT is an ideal methodology to use when little is known about a phenomenon (Glaser & Strauss, 1967). CGT allows the researcher to explore how a group of people define their reality and resolve their “main concern” (Glaser, 1998, p. 18).
The study was approved by the institutional review board of the University of Texas Medical Branch at Galveston. Data collection consisted of the informed consent process, demographic questions, and the interview. The interview used semistructured questions and prompts to encourage ongoing communication about the participant’s experiences (see Figure 1). 
CGT data analysis is an iterative process beginning with the first set of data and is guided by the synchronous, iterative techniques of constant comparative methodology (CCM), coding, memoing, and, ultimately, the emerging theory (Glaser, 1978, 1998). CCM is a data analysis strategy in which data are analyzed sentence by sentence, line by line; data items are compared to each other, then grouped into themes and categories, allowing emergence of the theory (Glaser, 1978). Coding the data allows the researcher to ask, “What is actually happening in the data?” (Glaser, 1978, p. 57), as well as to continue to do so until the main concern of the participants has been identified. The researcher distinguishes patterns among the categories and conceptualizes the relationships in the data for theory development (Glaser, 1998, 2005). Memoing permits the researcher to keep track of thoughts and ideas about the data, concepts, and emerging theory by a freestyle process of notemaking throughout the study.
Findings
The purpose of this CGT study was to explore what the OCTNs considered to be ethical challenges during the management of CTs and how the OCTNs resolved those conflicts. Data analysis led to the emergence of the OCTNs’ main concern, implementing an undefined job. The OCTNs resolved (Glaser, 1978) their main concern through the process of figuring it out.
Implementing an Undefined Job
Although the study participants were aware of Clinical Research Nursing: Scope and Standards of Practice (ANA & IACRN, 2016) and 2016 Oncology Clinical Trials Nurse Competencies (ONS, 2016), they did not find their position, in actual practice, to be clearly defined. They believed that these resources provided an overall ideal of the role and responsibilities of the CTN, but that the guidelines were not sufficiently specific to the unique situations they encountered in their daily practice. In addition, there were vast differences in how the participants managed their position because they found their daily duties to be unclear, ambiguous, and vague.
For example, the informed consent process widely varied by institution, department, and study participant regarding how individual patients were informed about the CT, as well as who provided the information about the CT, answered the patient’s questions, and obtained the patient’s agreement to participate. Some of the OCTNs did not participate in the informed consent process. Other OCTNs completed the entire consent process after the physician had introduced the CT and had exited the patient’s examination room, leaving the OCTNs with the responsibility of discussing the CT and obtaining the patient’s consent to participate. The OCTNs stated that they did not always feel competent enough to answer all the patient’s questions about the trial.
The OCTNs described ethical challenges they encountered as being “where something did not feel comfortable or right” (participant 2) and “gut-check scenarios” (participant 3). It was a situation with “no black-and-white answer” (participant 6) and “that makes you stop in your tracks” (participant 7). The OCTNs mentioned numerous examples of times when they were confronted with ethically challenging situations, such as issues with the informed consent process or problematic encounters with physicians or principal investigators.
In addition, the OCTNs met various demands and pressures they believed were, or could lead to, ethical issues, such as the specific requirements of each protocol, the principal investigators’ viewpoints on and expectations of the OCTNs, institutional and departmental policies, trial sponsor obligations and expectations, and unique patient situations (e.g., financial issues; social issues; physical issues, including those that could place the patient at risk during CT participation). These demands and pressures often competed with each other, creating confusing situations that the OCTNs were obligated to navigate. They considered these situations to be ethically challenging because there was a lack of clear standards they could follow during the actual day-to-day, patient-to-patient implementation of their job.
Figuring It Out
Figuring it out was how the OCTNs resolved their main concern of implementing an undefined job. According to one participant, “We just figured things out as we went along” (participant 11). When figuring it out, the OCTNs did what was necessary to protect their patient because patient safety always took precedence. When the OCTNs figured it out, they learned from their own experiences, resulting in a change in their practice as an OCTN. One OCTN described an encounter with a physician who wanted to continue a patient’s treatment on a CT, even though the patient’s disease was worsening. The OCTN learned that she had to be thoroughly prepared by knowing her protocol and comprehending all possible situations she might encounter. She stated that she changed her practice with that particular physician by always having the protocol with her to show to the physician, should another issue arise in the future.
Figuring it out consists of four strategies the OCTNs could apply to implement an undefined job: learning as they go, utilizing their assets, standing their ground, and managing hope.
Learning as they go: The study participants described inadequate training for their position that had left them unprepared to manage what they considered to be ethically challenging situations. As participant 2 reported, “Our training was on-the-go training.” Participants stated that they had received training specific to being an OCTN in a variety of ways, but found the training too short because the other OCTNs charged with educating them were also busy managing their own CTs: “All the nurses who are asked to help in the training process are so busy themselves. They do not have the time to cultivate an appropriate and successful training experience” (participant 9). The participants noted the tremendous support and encouragement they received from fellow OCTNs, even though they were busy managing their own patients who were taking part in CTs.
The OCTNs relied on other colleagues, research team members, principal investigators, and nurse managers for further guidance about their position. The OCTNs asked “a bazillion questions” (participant 9) to figure it out. One OCTN stated that she used the research protocol for her CT as her main resource; she also sought information in books, on the Internet, and by way of a lecture series to learn more about types of cancers, possible alternative treatments, and departmental and institutional policies. She feared she might give her patients the impression that she did not know what she was doing, so she used a variety of resources to gain confidence in her own judgment to make the right decisions and fulfill her responsibilities to her patients taking part in CTs.
Utilizing their assets: Generally, the OCTNs viewed themselves as the best resource for patients taking part in CTs. One participant said, “Nurses are vital to help the patients navigate the studies” (participant 5), and another said, “As the clinical trials nurse, I find myself as the go-to for the patient, whether it be protocol related or not” (participant 6). The OCTNs believed that they personalized and normalized the CT process for the patient by calling on their own nursing skills, such as patient assessment, education, and listening. Their proficiency in assessment and their knowledge of the patient allowed them to monitor the patient for side effects and implement nursing interventions or seek medical interventions. The OCTNs also used their skills related to patient education to encourage dialogue about possible side effects or the potential outcome of treatment. They made themselves aware of what the patients had been told by physicians about the possible side effects of the experimental treatment and believed that it was their obligation to clarify this information or provide additional education about the CT. In addition, they stressed the importance of their ability to listen to the patient, believing that forming relationships with patients gave them access to information about each patient’s situation that might affect their ability to participate in the CT. The OCTNs believed that their relationships with patients taking part in CTs, which were forged and nurtured over time, allowed them to deal with, or avoid entirely, ethical challenges that might arise during the CT.
The OCTNs also knew that, at times, they needed to call on the talents and knowledge of other people to assist the patient. According to one participant, “Since I’m usually the point of contact for patients, I try to do what I can to solve their problems or find someone that can help” (participant 8). The OCTNs understood the boundaries of the scope of their nursing practice, and they redirected questions to the treating physician, particularly during the process of obtaining the patient’s informed consent. In addition, the OCTNs recognized the principal investigator as an asset for helping the patient understand the CT because the principal investigator was “ultimately responsible” (participant 6) for the CT and for the participating patients.
Standing their ground: The OCTN’s first obligation is the safety of their patients. If the OCTNs believed that their patients’ safety was threatened in any way, they would stand their ground in the best interests of those patients or of the CT they managed. The OCTNs would also do so if they thought their patients were unable to provide informed consent on their own behalf. One OCTN described a situation where she was directed to obtain informed consent to participate in a CT from a patient who was waking up from sedation. She notified the physician that she refused to obtain consent because the patient was falling asleep when she was discussing the CT. The OCTN said she was reprimanded for delaying the patient’s treatment but that she believed her actions were in the patient’s best interest.
The OCTNs also described their obligation to protect the integrity of the protocols they managed. The OCTNs saw themselves as the gatekeeper for the protocol and would stand their ground if they believed the validity of the CT was threatened. Each protocol precisely defined the criteria the patient had to meet to enroll in that particular CT. Several OCTNs reported instances when physicians attempted to circumvent a protocol’s inclusion criteria so that a specific patient could enter that CT. In such instances, the OCTNs found it necessary to reassert the importance of the eligibility criteria to the physician: “Eligibility criteria [are] there for protection of the patient and the study data” (participant 8). The OCTNs would stand their ground even if doing so involved some personal risk to their position as a nurse because they were convinced that what they were doing was the right thing to do for their patient or for the integrity of the protocol.
Managing hope: The OCTNs described ethical challenges they encountered when they had to balance the reality of the patient’s diagnosis and the state of the patient’s disease with the possibilities offered by experimental treatment in a CT. Managing hope was usually uncomplicated for the OCTNs when they were interacting with patients with cancer seeking CT treatment for slow-growing, noninvasive cancers. The nature of their disease allowed such patients time to ask questions and consider whether treatment in a CT was their best treatment option. In contrast, OCTNs talked about the difficulty of managing hope with patients who were pursuing CT treatment as the last possible option to treat their disease. For these patients, all other treatment options available had failed, and they were eager to find an investigational treatment in a CT.
Part of managing hope for the OCTNs was ensuring that they were always honest with their patients, even if the conversations were difficult: “I always have to find right words and phrases to support the patient and not give them the wrong information” (participant 4). One OCTN said that because not all of the side effects of experimental treatment are known, “I have learned to be utterly honest about side effects” (participant 6). Generally, the OCTNs had positive experiences when they could offer hope with treatment available from a CT: “If I helped at least one patient who is on trial and doing well, I feel accomplished” (participant 4). In contrast, the experience could be emotionally taxing for the OCTNs if the patients were facing a poor outcome, despite treatment on a CT.
The OCTNs managed hope by understanding each patient’s situation, the patient’s goals for participating in the CT, and how to best support the patient in that decision. Their primary strategy for managing hope was to be truthful when talking to the patient, including being honest about treatment side effects and the treatment’s potential efficacy, and to support patients’ willingness to make the physical, financial, and time commitments required to participate in the CT.
Discussion
The OCTNs were knowledgeable about Clinical Research Nursing: Scope and Standards of Practice (ANA & IACRN, 2016) and 2016 Oncology Clinical Trials Nurse Competencies (ONS, 2016) as guidelines on how to perform their job. When the OCTNs were confronted with situations that they considered to be ethically challenging, they believed there was a lack of guidelines to address these situations. The OCTNs found that Clinical Research Nursing: Scope and Standards of Practice (ANA & IACRN, 2016) and 2016 Oncology Clinical Trials Nurse Competencies (ONS, 2016) only went so far; they believed that the daily activities of their position, particularly the specific and unique issues surrounding ethical challenges, could not be addressed in these publications because many of the challenges were patient or physician specific. The OCTNs saw it as their responsibility to immediately act when confronted with an ethically challenging situation or the prospect of an ethical challenge by diligently and creatively addressing each patient situation themselves. Because the OCTNs’ primary focus was patient safety, they figured out how to implement an undefined job when confronted with an actual or potentially ethically challenging situation by learning from their own experiences and applying this knowledge to future situations in an effort to better understand how to manage the ethical challenge, should it occur again.
When the OCTNs defined the term “ethical challenge,” they described any situation that might threaten the safety or welfare of the patient taking part in a CT, or a situation that jeopardized the quality of the data or the integrity of the CT. Many of the incidents the OCTNs described as ethically challenging echoed encounters discussed in the literature surrounding the areas of informed consent and protocol compliance (Cantini & Ells, 2007; Cisar & Bell, 1995; McEvoy et al., 1991).
Most of the ethically challenging situations the OCTNs described actually had occurred in their professional practice. The OCTNs also discussed other ethically challenging situations that could have occurred if the OCTN had not intervened. Their actions, designed to deal with or prevent an ethically challenging situation, revealed the OCTNs’ courage in the face of threats to their patients’ safety or the CT protocols’ integrity. The OCTNs placed the welfare of their patients and the CT ahead of personal risk to their position as a nurse.
The current study highlighted the education and preparation of the OCTN and gave insight into the need for novice OCTNs to have the basic knowledge and skills to fulfill the responsibilities of their new role before being assigned to manage research protocols and patients independently. The overall consensus of the OCTNs was that there was a lack of training for their position and that the training varied from institution to institution and from department to department. To a great extent, the OCTNs used their resources by accessing their own nursing knowledge, the knowledge of others, and the guidance of the protocol or their professional colleagues.
The theory of implementing an undefined job reflects the OCTNs’ determination to do what was best for their patients, even when how they should implement their job was not clear. They learned from their experiences and applied this knowledge to future situations in an effort to better manage ethical challenges.
Limitations
Study limitations included the study sample. The 12 nurse participants were self-selected; therefore, the study results reflect only the opinions and experiences of those OCTNs. The business cards and website used to recruit participants asked OCTNs to share their experiences about ethical challenges in practice, which might have limited a more diverse group of participants willing to share their personal experiences. In addition, the study examined only the specialty practice of OCTNs, which might limit generalizability of the study findings.
Implications for Nursing
The theory of implementing an undefined job enhances the literature about OCTNs and reveals the OCTNs’ commitment to their patients’ safety and their dedication to providing quality care to patients taking part in a CT. The study also highlighted the gap between guidance provided by Clinical Research Nursing: Scope and Standards of Practice (ANA & IACRN, 2016) and 2016 Oncology Clinical Trials Nurse Competencies (ONS, 2016) and the realities met by the OCTNs in their day-to-day, patient-to-patient encounters. The primary implications for nursing revealed by the study were the OCTNs’ need for enhanced, ongoing training and education for the qualities that make OCTNs successful in their role.
Several strategies could be implemented to meet OCTNs’ need for enhanced education, possibly beginning with a more standardized curriculum or educational program for OCTNs. Novice OCTNs also should be mentored by an experienced OCTN whose workload includes time that they can be available to provide guidance and a meaningful training experience. Institutions could provide educators to oversee the novice OCTNs’ training and additional education for experienced OCTNs, as well as facilitate ongoing debriefing sessions for all OCTNs who experience ethical challenges in practice. In addition, institutions or departments should create libraries of resources for OCTNs to reference when navigating the day-to-day, patient-to-patient implementation of their role.
The OCTNs who participated in the current study demonstrated commitment, ingenuity, and courage in finding ways to meet the needs of their patients taking part in CTs. Their obligation to the integrity of the CT they managed was one way that they fulfilled dedication to their patients. 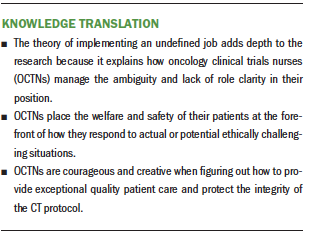
Conclusion
This CGT (Glaser, 1978, 1998) study explored ethical challenges experienced by OCTNs in their professional practice. The theory of implementing an undefined job emerged from the study findings. The OCTNs figured out how to implement their undefined job using the strategies of learning as they go, utilizing their assets, standing their ground, and managing hope. This theory has implications for the professional practice and education of OCTNs and highlights the commitment of OCTNs to the welfare of their patients taking part in CTs and to the CT protocol itself.
About the Author(s)
Sheryl G. Forbes, PhD, MEd, RN, CCRP, is a research quality nurse specialist in the Department of Nursing Administration at the University of Texas MD Anderson Cancer Center in Houston, and Carolyn A. Phillips, RN, PhD, is an associate professor and the Dibrell Family Professor in the Art of Medicine in the School of Nursing and in the Graduate School of Biomedical Sciences at the University of Texas Medical Branch at Galveston, all in Texas. No financial relationships to disclose. Both authors contributed to the conceptualization, design, and manuscript preparation. Forbes completed the data collection, and provided statistical support and analysis. Forbes can be reached at sheryl.g.forbes@gmail.com, with copy to ONFEditor@ons.org. (Submitted August 2019. Accepted for publication January 18, 2020.)




