Surface Contamination With Antineoplastic Drugs on Two Inpatient Oncology Units
Objectives: To measure surface contamination with antineoplastic drugs on inpatient oncology units and to characterize nursing staff personal protective equipment (PPE) use and factors that predict this use.
Sample & Setting: A descriptive pilot study of two inpatient oncology units at Duke University Hospital in Durham, North Carolina, administering etoposide and cyclophosphamide.
Methods & Variables: Surfaces in four patient rooms and select shared areas were swabbed with methanol, acetonitrile, and water. Samples were analyzed by liquid chromatography tandem mass spectrometry. Nursing staff (N = 27) answered questions about their demographics, PPE use, and factors that influence PPE use via online survey.
Results: Contamination with cyclophosphamide and etoposide was detectable and quantifiable in 61% and 31% of surfaces tested, respectively. Nursing staff reported suboptimal use of PPE when administering, disposing, and handling excreta of patients. Workplace safety climate was predictive of PPE use.
Implications for Nursing: The potential for contamination with antineoplastic drugs in inpatient oncology units presents exposure risks for healthcare workers, patients, family members, and visitors. Future research and interventions to limit exposure and increase routine surface sampling should focus on those areas of greatest contamination, including toilet seats, a prominent finding from the current study.
Jump to a section
Antineoplastic drugs (ADs) are among the most toxic of the hazardous drugs administered in healthcare settings. Even low levels of AD contamination put healthcare workers at risk for genotoxicity, carcinogenicity, teratogenicity, fertility impairment, reproductive toxicity, and/or serious organ toxicity from repeated exposures to multiple drugs (Boiano et al., 2014; National Institute for Occupational Safety and Health [NIOSH], 2004; Suspiro & Prista, 2011). Whereas inhalation, ingestion, injection, and even ocular exposure are possible, dermal exposure is the most common route of entry for healthcare workers. Dermal exposure may occur through direct contact with the AD or indirectly through surfaces contaminated with the AD.
Data on surface contamination exist, but there are still gaps in knowledge. First, there is currently no acceptable limit for AD surface contamination; rather, it should be as low as reasonably achievable (Suspiro & Prista, 2011; U.S. Pharmacopeia [USP], 2019). Second, there is a limited understanding of the areas that are most at risk for surface contamination. Most published data on surface contamination have been collected in outpatient oncology administration areas or pharmacy compounding areas (Kopp et al., 2013; Maeda et al., 2010; Salch et al., 2019; Yoshida et al., 2009). The extent of surface contamination in inpatient oncology areas, including patient rooms and shared areas, has been less studied. In addition, some studies grouped all shared and patient areas together, making it difficult to ascertain the most contaminated ones (Bussiéres et al., 2012; Connor et al., 2010, 2016; Janes et al., 2015). Understanding which surfaces are the most likely to be contaminated in patient rooms and in shared areas (surfaces where the medications are not administered and where employees are unlikely to be wearing personal protective equipment [PPE]) is needed.
Policies and recommendations exist to limit healthcare workers’ exposure to hazardous drugs (NIOSH, 2004; Polovich & Olsen, 2018; Power & Coyne, 2018). As of December 1, 2019, wipe sampling is recommended as a measure of containment (USP, 2019). Per the NIOSH (2015) hierarchy of controls, the most effective controls in the hierarchy (elimination and substitution) are not feasible because of the therapeutic benefits of the drugs to patients. Therefore, to protect healthcare workers, focusing on administrative controls and PPE is necessary. Employers must train employees to use PPE (individual controls), as well as educate them on exposure risks and assess their competency annually (administrative controls). Prior research has demonstrated that exposure potential persists because of inadequate training, suboptimal use of PPE, and perception of a suboptimal workplace safety climate (Boiano et al., 2014; Silver et al., 2016).
The aims of this study were to describe inpatient oncology surfaces most contaminated with ADs and to characterize PPE use and factors that predict its use among inpatient oncology staff. The authors conducted a pilot study that included wipe sampling of surfaces in inpatient oncology settings (in patient rooms and shared areas). Unit staff were surveyed on self-reported PPE use and factors that theoretically predict that PPE use, as well as questions about orientation and annual refreshers.
Methods
Sample and Setting
This study took place on two inpatient units—one for medical oncology and one for bone marrow transplantation—at Duke University Hospital in Durham, North Carolina, which specializes in providing care to adults with hematologic malignancies. Both units provided chemotherapy to as many as 10 patients per day. After the Duke Medicine Institutional Review Board reviewed the study and determined it as exempt from human subjects review, the authors obtained verbal consent from patients to enter their rooms and sample surfaces for AD contamination.
Surface Sampling
Shared surfaces are located in areas where patients are not receiving ADs and where a variety of healthcare workers, family members, and patients may come into contact with surfaces. It is not expected that PPE would be used in shared areas. Eighteen surfaces in shared areas on each of the two units were sampled on two different days. Seventeen surfaces in each of four rooms of patients receiving either cyclophosphamide or etoposide were also sampled. Shared and room surfaces were chosen after an examination of the literature (Connor et al., 2016), a conversation with infection control, and a consideration of resource availability. Samples were collected from surfaces (average surface area = 180 cm2) composed of plastic, metal, linoleum, and laminated composite material (e.g., Formica®). Disposable, pre-measured templates were used to define the areas to be sampled. Surfaces were systematically wiped by the first author using sampling swabs dipped in a solution of 10% acetonitrile, 25% methanol, and 65% deionized water (pH = 6) (Connor & Smith, 2016). Wipes were collected in 5 ml polypropylene tubes and stored in a –20ºC freezer until analysis.
Drug Recovery Validation
To test the efficacy of drug wipeoff from surfaces and subsequent drug extraction from wipes, cyclophosphamide and etoposide were prepared in their clinical formulations and 5 mcl of low and high concentration (0.4 ng/mcl and 2,000 ng/mcl for cyclophosphamide; 0.4 ng/mcl and 400 ng/mcl for etoposide) of each drug was pipetted onto four different surfaces (measuring 200 cm2): polypropylene, laminated composite material (formaldehyde resin), waxed (polyurethane) vinyl floor, and stainless steel. This resulted in the applied amounts of 0.01 ng/cm2 and 50 ng/cm2 for cyclophosphamide and 0.01 ng/cm2 and 10 ng/cm2 for etoposide. The surfaces were left to dry overnight at room temperature. For cyclophosphamide, resulting average recovery of the low concentration was 132% (SD = 34%) and of the high concentration was 95% (SD = 39%). For etoposide, resulting average recovery of the low concentration was 74% (SD = 26%), and of the high concentration was 74% (SD = 12%). The satisfactory recovery of trace amounts of drug from all surface types allowed focus on the areas of greatest contamination despite type of surface.
Drug Extraction
Drug extraction solution (5 ml) of 50% acetonitrile, 50% methanol, and 1 ng/ml each of cyclophosphamide-d4 and etoposide-d3 (isotopically labeled internal standards) were added to sample tubes. After rotary agitation for 10 minutes, samples were stored at –20ºC until analysis. On the day of analysis, 100 mcl of the sample (extraction solution) was mixed with 100 mcl of mobile phase A, and 50 mcl was injected into the liquid chromatography tandem mass spectrometry system.
Data Analysis
Liquid chromatography tandem mass spectrometry was used to analyze the samples. Shimadzu 20A series liquid chromatography and Applied Biosystems/SCIEX API 5500 QTrap tandem mass spectrometry system was used for quantitative analysis. Liquid chromatography conditions were as follows:
• Agilent eclipse 4.6 x 30 mm column
• Mobile phase A: 10 mm ammonium acetate, 0.1% formic acid, 3% acetonitrile in water
• Mobile phase B: 50% acetonitrile, 50% methanol; elution gradient: 0–1 minutes 20%–70% B, 1–1.3 minutes 70%–20% B
• Tandem mass spectrometry transitions used for quantification (injection volume): 261/139.9 (cyclophosphamide), 265/139.9 (cyclophosphamide-d4), 606.2/229 (etoposide), and 609.2/229 (etoposide-d3)
Additional transitions were also followed for identification (qualification) purposes. Calibration samples for cyclophosphamide and etoposide in the 0.037–3 ng/ml range were analyzed alongside the study samples. The lower level of quantification for cyclophosphamide and etoposide was 0.037 ng/ml (80% accuracy criterion). Results were expressed as ng/ml of recovery solvent and then converted to ng/cm2 based on surface area of the site sampled. Those conducting the analyses were blinded to the site where sampling occurred.
To standardize reporting of results, Connor et al. (2016) recommend that surface contamination be reported in ng/cm2. Samples were characterized as not detectable, detectable but not quantifiable, and detectable and quantifiable. The detectable and quantifiable group was further categorized as 0.0–0.05 ng/cm2; 0.051–0.1 ng/cm2 (0.05 is considered a detectable level by some commercial testing companies); and greater than 0.1 ng/cm2, indicating a relatively high level of contamination.
Online Survey
Individual participant data were collected from nursing staff via a REDCap (Harris et al., 2009) survey using the Revised Hazardous Drug Handling Questionnaire (Polovich & Clark, 2012). The instrument measures self-reported precaution use, as well as several predictor variables. Precaution use is measured on a six-point Likert-type scale ranging from 0 (never) to 5 (always) during HD administration, disposal, and handling of HD-contaminated excreta. Total precaution use is the mean of the scores for the 17 items, indicating the frequency of precaution use.
Predictor variables were measured, and some were reverse-scored so that higher scores indicate higher presence of the predictor. The variables of interest were barriers to using PPE (13 items; five-point Likert-type scale from 0 [strongly disagree] to 4 [strongly agree]), perceived risks of chemotherapy exposure (3 items; four-point Likert-type scale from 1 [strongly disagree] to 4 [strongly agree]), workplace safety climate (21 items; five-point Likert-type scale from 1 [strongly disagree] to 5 [strongly agree]), and perceived conflict of interest (6 items; four-point Likert-type scale from 1 [strongly disagree] to 4 [strongly agree]). Interpersonal influence on precaution use was measured by two subscales: interpersonal norms (four items measuring the importance to others of using PPE on a three-point Likert-type scale from 0 [not at all] to 2 [very]) and interpersonal modeling (three items measuring frequency of PPE use by others on a four-point Likert-type scale from 0 [never] to 3 [usually]). Construct validity of the predictor variables was supported by the strength and direction of the relationships between the predictor variables (except for perceived conflict of interest) and total precaution use (Polovich & Clark, 2012). Factor analysis further supported the construct validity of the Workplace Safety Climate subscale (Gershon et al., 2000) and the Barriers to Using PPE subscale (Polovich & Clark, n.d.). Internal consistency reliability (Cronbach alpha) for the subscales measuring the predictor variables ranged from 0.7 to 0.95 in nurses, and test-retest reliability ranged from 0.7 to 0.92 (Polovich & Clark, 2012). Demographic questions included years of experience and type and recency of training. The survey took about 20 minutes to complete and was open for four weeks. The survey was sent to all nursing and nursing assistant staff on unit 1 but only to nursing staff on unit 2 at the discretion of the nurse manager. Participants were compensated with bagels or pizza at four points during the study at the unit level. There was no individual remuneration for survey participation. Staff received the anonymized results of the study at a unit staff meeting.
Statistical Analysis
AD contamination was summarized by the number and percentage of surfaces with AD residue. The data were categorized by level of detection for the two drugs. Controlling for the unit, location, and interaction between the two, a multilevel model was fit. The least means estimated unit-location interaction, and overall unit effects were compared. The survey data were analyzed by descriptive statistics for the demographic variables. T tests and chi-square tests were conducted to compare results by unit. Simple linear regression was performed to examine bivariate associations between total PPE use and each of the predictive factor domains separately. For the predictive domains that were significant, the authors examined the items within that factor on total PPE use. All analyses were conducted in SAS, version 9.4, and significance level of 0.05 was used to determine significance for all inferential tests.
Results
Surface Contamination
Contamination with cyclophosphamide was detectable and quantifiable in 86 of 140 (61%) surfaces tested (see Table 1). Levels greater than 0.05 ng/cm2 (a level commonly reported in commercial wipe testing) were found on 22 of 140 (16%) surfaces. Contamination with etoposide was detectable and quantifiable in 43 of 139 (31%) surfaces tested. Levels greater than 0.05 ng/cm2 were found on 9 of 139 (6%) surfaces tested. A larger number of surfaces in patient rooms were detectable and quantifiable, and higher levels of contamination occurred in patient rooms than in shared areas (see Table 2). Contamination was found at detectable and quantifiable levels for all surface types (metal, plastic, linoleum) except laminated composite material.
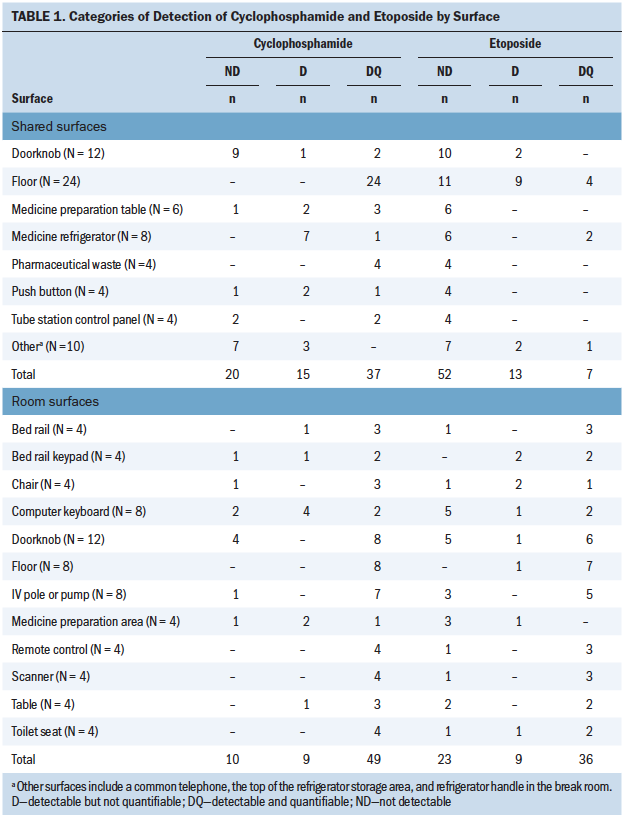
Shared Areas
The most contaminated shared locations were the floors, including floors near the pharmaceutical waste bin; common desks; and in personnel lounges, bathrooms, and locker rooms. Other shared locations that were commonly contaminated included medication refrigerator handles and common telephones.
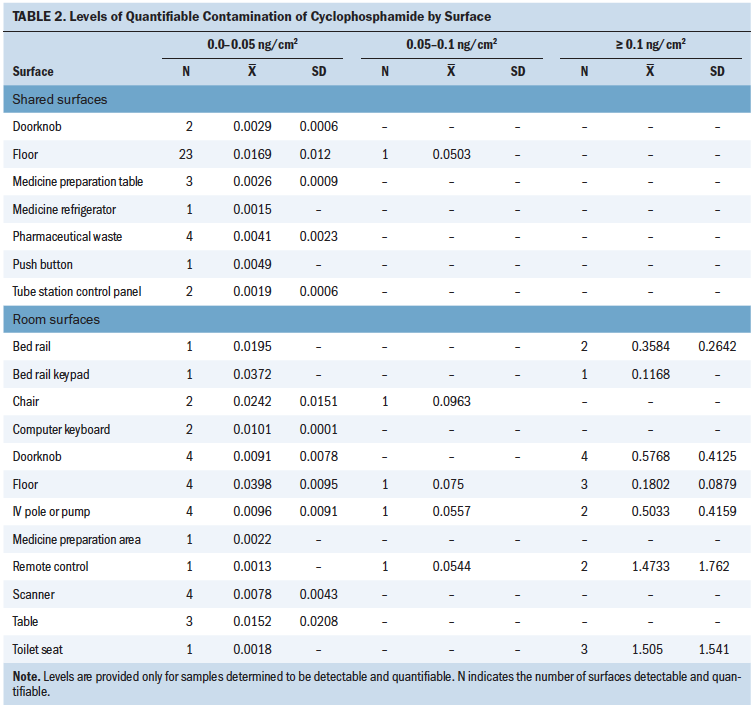
Patient Room Areas
The most contaminated in-room locations included toilet seats, floors near the IV pole during drug administration, remote controls, doorknobs (to enter the room and the restroom), bed rails, IV poles or pumps, computer keyboards, and scanners. In addition, more contamination occurred in patient rooms with cyclophosphamide on unit 1 than on unit 2. In one-third of the patient room surfaces tested in which the current patient received only one drug of interest, the other drug was also found (17 of 51 surfaces).
Online Survey
Nurses (n = 25) and nursing assistants (n = 2) completed the online survey (73% completion rate). Table 3 provides the demographic characteristics of the nursing staff. All participants come into contact with body fluids and/or linens of patients receiving chemotherapy as part of their jobs. Of note, orientation at the start of the job and annual training about AD safe handling were provided. Twenty-six of 27 employees reported orientation and 24 of 27 employees reported receiving an annual refresher about safe handling of chemotherapy at their workplace. Respondents on unit 1 reported a higher number of patients receiving chemotherapy per day and a larger number of patients that they personally cared for receiving chemotherapy. There were also differences in self-reported PPE use. Unit 1 had more contamination with cyclophosphamide, administered more doses of the drug, had a higher volume of patients, and had less use of PPE except for plastic-backed pads while flushing. Staff on unit 2 reported more use of masks during administration, disposal, and handling contaminated excreta, and more use of double gloves and gowns during administration of ADs. On unit 1, the median number of years of chemotherapy-handling experience was 13 (interquartile range [IQR] = 2, 19); on unit 2, the median number was 3 (IQR = 1, 9) (p = 0.167).
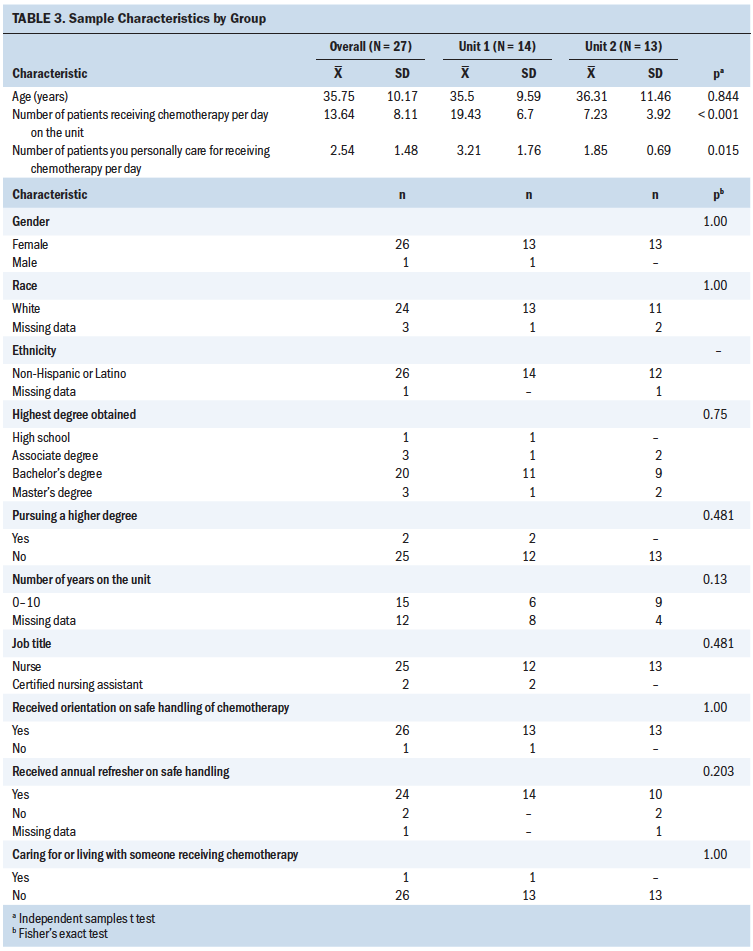
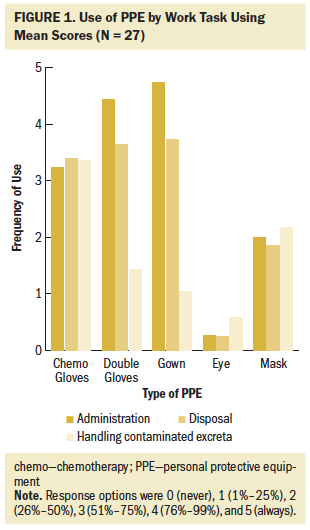
Staff reported use of PPE when administering, disposing of, and handling excreta of patients receiving these drugs, but there was room for improvement in use of all PPE during all work tasks (see Figure 1). During administration, wearing chemotherapy gowns had the highest adherence, followed by use of double gloves. This is also true for drug disposal. For handling contaminated excreta, the highest-ranking behavior was gloves at 3.36 (maximum = 5); however, the distribution is very wide (SD = 2.31). Safe handling occurs more in the administration of ADs (mean = 2.81 for all PPE use and protective behavior combined) than in disposal (mean = 2.62 combined) or handling contaminated excreta (mean = 1.77 combined), with the overall mean score trailing nearly one unit below the means for administration and disposal of ADs. Eye protection, regardless of work task, scored as almost never despite its recommended use any time splashing is possible. Of note, the mean for use of closed-system transfer devices (CSTDs) during administration was 1.79, and the mean for use of plastic-backed pads while flushing during the handling of AD-contaminated excreta was 1.77.
The only factor of influence significantly associated with the total protective behaviors (defined as the mean of the 17 items for administration, disposal, and handling contaminated excreta) was workplace safety climate (r = 0.46, p ≤ 0.05). For each one-point increase in workplace safety climate score (maximum score = 105), total protective behaviors were expected to increase by 0.03. That is, someone who reported their workplace safety climate as 5 points safer than another participant would be likely to have a protective behavior score that was 0.15 points higher as well, making those who perceive their workplaces as more oriented toward safety also more likely to personally practice safety behaviors. However, when the factors were examined against overall protective behaviors by unit, there were no significant associations on unit 1. On unit 2, two moderate associations emerged: self-efficacy (r = 0.59, p ≤ 0.05) and workplace safety climate (r = 0.82, p ≤ 0.05). These results must be interpreted with caution because the sample sizes were small; the authors had 51% power to detect a minimum correlation of 0.707 for unit 1 (n = 14) and 47% power to detect a minimum correlation of 0.707 for unit 2 (n = 13).
Workplace safety climate had 21 individual items, and the authors analyzed those separately because they may be critical to future interventions. The authors found that the three questions that predicted the majority of the variance within workplace safety climate were as follows:
• Correction of unsafe work practices by a supervisor (effect size = 24.4%, F = 8.06, p = 0.008)
• Accessibility of chemotherapy-rated gloves (effect size = 20.5%, F = 6.45, p = 0.018)
• Having an uncluttered work area (effect size = 18.9%, F = 5.61, p = 0.026)
Discussion
Despite recommendations and policies to minimize exposure to ADs for healthcare workers, surface contamination persists in patient administration areas, as well as shared areas where nursing staff, patients, and families interact without wearing PPE. The fact that many samples were detectable but not quantifiable; or if quantifiable, then at a level below the level of detection set by commercial companies, speaks to the sensitivity of the assay and analytical technique. The authors obtained data that helps prioritize where to look for surface contamination as a measure of containment in inpatient administration areas, as is recommended by USP General Chapter <800>. The current study demonstrates that patient administration surfaces are more contaminated than shared areas. Toilet seats, handheld objects in patient rooms, doorknobs, and floors in patient rooms, as well as floors and handles in shared areas, should be considered surfaces to sample. Toilet seats are an underconsidered source of exposure to ADs in inpatient settings, which has implications for all healthcare workers, particularly nursing assistants and environmental services workers, who have some of the lowest levels of formal education and training (Walton et al., 2019).
The contamination on handheld items suggests the importance of reinforcing the doffing of PPE. It is not surprising that contamination occurs in areas where ADs are not being administered. However, the fact that floors were the most contaminated shared area suggests a potential mechanism for tracking those agents around the healthcare facility and supports a finding about high levels of contamination on floors from a prior study (Connor et al., 2010). In addition, staff should be asked if they are wearing their work shoes at home, and staff education should include a recommendation to avoid wearing work shoes around the home.
The current findings also have implications for patients and caregivers in the home. Often, patients are discharged within the first 48 hours of chemotherapy administration but may excrete these drugs for up to seven days after administration, depending on the drug, the metabolism of the individual, and other factors (Polovich & Olsen, 2018). The authors learned that drugs can be excreted unchanged and may serve as a significant source of contamination. The other potential mechanism for the drug on the toilet seat would be disposal of the drug in the toilet. However, the authors do not believe that this happened, but it could be asked in a future study. These findings emphasize the need for education about the tasks that have exposure potential. They also lead to questions about the efficacy of plastic-backed pads to reduce exposure from AD-contaminated excreta.
In one-third of the in-room surfaces tested, a drug was found that was not administered, which gives reason to consider more carefully how cleaning is done in rooms between patients and how equipment is or is not shared between rooms. A four-step process is needed to clear ADs from surfaces, including deactivating, decontaminating, disinfecting, and removing (USP, 2019). However, the effectiveness of the “discharge clean” is not known; it is also not known if cross-contamination may occur when the nurse administers multiple drugs to multiple patients or when other healthcare personnel work between rooms. Better understanding of the cleaning process through observation is critical to minimize exposures.
Suboptimal use of PPE has been reported in prior studies as well (Graeve et al., 2017; Polovich & Clark, 2012). However, this is one of only a few studies to specifically assess use of plastic-backed pads when flushing the toilet as a means to minimize exposure to ADs in excreta. The staff responding to the survey reported using plastic-backed pads when flushing the toilet about 50% of the time, and unit 1 reported using more of these than unit 2. In a prior study with nursing assistants, this behavior was overreported when compared to observed (Walton et al., 2019). Whereas use of masks was high on unit 2, it is worth noting that it is a bone marrow transplantation unit where staff wear masks as part of universal precautions for immunocompromised patients. In the case of minimizing exposure to ADs, masks are used to provide splash protection; they are insufficient for respiratory protection. The situation in non-oncology units where these drugs are given less frequently and where none of these standard precautions are in place is unclear. This study does not include many nursing assistants, and the authors cannot differentiate what they do in regard to PPE use when coming into contact with AD-contaminated excreta. Prior work with nursing assistants suggests that they are highly influenced by the PPE use of nurses (Walton et al., 2019). It has also demonstrated that they desire more standardized education and training regarding safe handling of ADs (Walton et al., 2019). Finally, staff reported using CSTDs about 50% of the time when administering ADs when the facility had no CSTDs available on the unit, demonstrating opportunities for education about these devices. When the authors presented findings to study participants, they asked what participants thought was meant by CSTDs, and responses included preprimed tubing and Luer locks. It also reinforces concerns about the validity of self-reported data and suggests the need for observational data (Walton et al., 2019).
The only factor of influence moderately associated with PPE use was workplace safety climate. Workplace safety climate has emerged in prior studies as associated with PPE use (Polovich & Clark, 2012). The findings lead the authors to consider a focus on nursing supervisors, accessibility of chemotherapy-rated gloves, and decluttering of the work area as potential targets for intervention. Nursing supervisors have control over availability of PPE, ability to enforce the practice of protective behaviors, and ability to shape the safety culture on the unit. Decluttering of the work area has been proven beneficial in a multitude of settings and has been a focus of recent efforts in the healthcare industry to reduce waste and increase efficiency. The current study illuminates that more research is needed on AD-contaminated excreta as a significant source of exposure for healthcare workers and on examination of discharge cleaning methods to remove ADs.
Limitations
This study has a few limitations. First, this pilot study was conducted on two inpatient units at the same hospital, and findings may not be reflective of other hospitals or outpatient areas in the same medical center. Second, the number of respondents was small and did not include nursing assistants on one of the units. Third, data on PPE use were self-reported and not based on observation. Fourth, the authors only included two drugs; however, the drugs chosen are very commonly reported in the literature and used in practice. The authors also had developed highly sensitive assays for the drugs used. The authors did not detect contamination on laminated composite material on the units, but there were few surfaces in the study composed of that material.
Implications for Nursing and Research
Nurses should be aware that surface contamination persists, even in shared areas where patients are not receiving the agents and where nurses are not customarily wearing PPE. Nurses may be surprised to learn that the most contaminated areas on the units were toilet seats. In addition, ADs that were not administered to the current patient were still found on surfaces in patient rooms. Nurses may benefit from reflection on their suboptimal use of PPE in the context of that contamination. Nurses need to consider that AD-contaminated excreta may be a source of exposure for nursing assistants and environmental services workers. Nurses have the opportunity to model proper use of PPE, as well as to participate in formal education of their colleagues. Nurses make up the majority of the workforce on these inpatient units and need to take the lead for education about workplace safety for all. Workplace safety climate and interventions that include nurse managers in particular may be an important focus for future study and intervention to increase the practice of protective behaviors.
Further research is needed about disposal of AD-contaminated excreta, routine cleaning, and decontamination of areas where ADs are administered. Residue of ADs in patient rooms when the current patients did not receive those drugs indicates that contamination persists despite cleaning. In inpatient areas, there is no recommended frequency for surface decontamination. Such information is essential to minimizing exposure for personnel, visitors, and patients. The consideration of AD-contaminated excreta as a source of exposure, also lends itself well to greater inclusion of nursing assistants in research, as well as research questions regarding PPE use and exposures of family caregivers in the home setting. 
Conclusion
Contamination with ADs persists in inpatient oncology administration areas and in shared areas where patients, family, and staff are not wearing PPE. The current study suggests locations that are likely contaminated. Toilets emerged as the surface most contaminated with ADs. More research on understanding the contribution of AD-contaminated excreta is needed. In addition, the fact that drugs not administered in a patient’s room were found in the room demonstrates the need to examine the efficacy of discharge cleaning methods. Nursing staff in this study and in most others have used PPE suboptimally; workplace safety climate may be an important focus of intervention for increasing PPE use, particularly among nursing staff.
About the Author(s)
AnnMarie Lee Walton, PhD, MPH, RN, OCN®, CHES, is an assistant professor and Margaret A. Bush, PhD, MBA, BCOP, is an associate professor, both in the School of Nursing at Duke University in Durham, NC; Christian Elizabeth Douglas, DrPH, is a research biostatistician at Social and Scientific Systems in Chapel Hill, NC; Deborah H. Allen, PhD, RN, CNS, FNP-BC, AOCNP®, is the director of nursing research and evidence-based practice in the Duke University Health System in Durham, NC; Martha Polovich, PhD, RN, is a part-time instructor in the Byrdine F. College of Nursing and Health Professions at Georgia State University in Atlanta; and Ivan Spasojevic, PhD, is an associate professor in the Department of Medicine–Oncology at Duke University and the director in the Pharmacokinetics/Pharmacodynamics Core Laboratory in the School of Medicine at Duke Cancer Institute in Durham, NC. Walton, Bush, Allen, Polovich, and Spasojevic contributed to the conceptualization and design. Walton and Bush completed the data collection. Walton, Bush, Douglas, and Spasojevic provided analysis. Douglas provided statistical support. All authors contributed to the manuscript preparation. Walton can be reached at annmarie.walton@duke.edu, with copy to ONFEditor@ons.org. (Submitted September 2019. Accepted for publication December 6, 2019.)
References
Boiano, J.M., Steege, A.L., & Sweeney, M.H. (2014). Adherence to safe handling guidelines by health care workers who administer antineoplastic drugs. Journal of Occupational and Environmental Hygiene, 11(11), 728–740. https://doi.org/10.1080/15459624.2014.916809
Bussiéres, J.F., Tanguay, C., Touzin, K., Langlois, É., & Lefebvre, M. (2012). Environmental contamination with hazardous drugs in Quebec hospitals. Canadian Journal of Hospital Pharmacy, 65(6), 428–435. https://doi.org/10.4212/cjhp.v65i6.1190
Connor, T.H., DeBord, D.G., Pretty, J.R., Oliver, M.S., Roth, T.S., Lees, P.S.J., . . . McDiarmid, M.A. (2010). Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. Journal of Occupational and Environmental Medicine, 52(10), 1019–1027. https://doi.org/10.1097/JOM.0b013e3181f72b63
Connor, T.H., & Smith, J.P. (2016). New approaches to wipe sampling methods for antineoplastic and other hazardous drugs in healthcare settings. Pharmaceutical Technology in Hospital Pharmacy, 1(3), 107–114. https://doi.org/10.1515/pthp-2016-0009
Connor, T.H., Zock, M.D., & Snow, A.H. (2016). Surface wipe sampling for antineoplastic (chemotherapy) and other hazardous drug residue in healthcare settings: Methodology and recommendations. Journal of Occupational and Environmental Hygiene, 13(9), 658–667. https://doi.org/10.1080/15459624.2016.1165912
Gershon, R.R.M., Karkashian, C.D., Grosch, J.W., Murphy, L.R., Escamilla-Cejudo, A., Flanagan, P.A., . . . Martin, L. (2000). Hospital safety climate and its relationship with safe work practices and workplace exposure incidents. American Journal of Infection Control, 28(3), 211–221. https://doi.org/10.1067/mic.2000.105288
Graeve, C., McGovern, P.M., Arnold, S., & Polovich, M. (2017). Testing an intervention to decrease healthcare workers’ exposure to antineoplastic agents. Oncology Nursing Forum, 44(1), E10–E19. https://doi.org/10.1188/17.ONF.E10-E19
Harris, P.A., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J.G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Janes, A., Tanguay, C., Caron, N.J., & Bussiéres, J.F. (2015). Environmental contamination with cyclophosphamide, ifosfamide, and methotrexate: A study of 51 Canadian centres. Canadian Journal of Hospital Pharmacy, 68(4), 279–289.
Kopp, B., Schierl, R., & Nowak, D. (2013). Evaluation of working practices and surface contamination with antineoplastic drugs in outpatient oncology health care settings. International Archives of Occupational and Environmental Health, 86(1), 47–55. https://doi.org/10.1007/s00420-012-0742-z
Maeda, S., Miyawaki, K., Matsumoto, S., Oishi, M., Miwa, Y., & Kurokawa, N. (2010). Evaluation of environmental contaminations and occupational exposures involved in preparation of chemotherapeutic drugs. Yakugaku Zasshi, 130(6), 903–910. https://doi.org/10.1248/yakushi.130.903
National Institute for Occupational Safety and Health. (2004). NIOSH alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in health care settings (DHHS [NIOSH] Publication No. 2204-165). U.S. Department of Health and Human Services, Centers for Disease Control. https://www.cdc.gov/niosh/docs/2004-165/pdfs/2004-165.pdf
National Institute for Occupational Safety and Health. (2015). Hierarchy of controls. https://www.cdc.gov/niosh/topics/hierarchy
Polovich, M., & Clark, P.C. (2012). Factors influencing oncology nurses’ use of hazardous drug safe-handling precautions. Oncology Nursing Forum, 39(3), E299–E309. https://doi.org/10.1188/12.ONF.e299-e309
Polovich, M., & Clark, P.C. (n.d.). [Construct validity of the Barriers to Using PPE subscale]. Unpublished raw data.
Polovich, M., & Olsen, M.M. (Eds.). (2018). Safe handling of hazardous drugs (3rd ed.). Oncology Nursing Society.
Power, L.A., & Coyne, J.W. (2018). ASHP guidelines on handling hazardous drugs. American Journal of Health-System Pharmacy, 75(24), 1996–2031. https://doi.org/10.2146/ajhp180564
Salch, S.A., Zamboni, W.C., Zamboni, B.A., & Eckel. S.F. (2019). Patterns and characteristics associated with surface contamination of hazardous drugs in hospital pharmacies. American Journal of Health-System Pharmacy, 76(9), 591–598. https://doi.org/10.1093/ajhp/zxz033
Silver, S.R., Steege, A.L., & Boiano, J.M. (2016). Predictors of adherence to safe handling practices for antineoplastic drugs: A survey of hospital nurses. Journal of Occupational and Environmental Hygiene, 13(3), 203–212. https://doi.org/10.1080/15459624.2015.1091963
Suspiro, A., & Prista, J. (2011). Biomarkers of occupational exposure do anticancer agents: A minireview. Toxicology Letters, 207(1), 42–52. https://doi.org/10.1016/j.toxlet.2011.08.022
U.S. Pharmacopeia. (2019). USP General Chapter <800> hazardous drugs—Handling in healthcare settings. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handlin…
Walton, A., Kneipp, S., Linnan, L., Asafu-Adjei, J., Douglas, C., Leff, M., & Rogers, B. (2019). Nursing assistants’ use of personal protective equipment regarding contact with excreta contaminated with antineoplastc drugs. Oncology Nursing Forum, 46(6), 689–700. https://doi.org/10.1188/19.ONF.689-700
Yoshida, J., Tei, G., Mochizuki, C., Masu, Y., Koda, S., & Kumagai, S. (2009). Use of a closed system device to reduce occupational contamination and exposure to antineoplastic drugs in the hospital work environment. Annals of Occupational Hygiene, 53(2), 153–160. https://doi.org/10.1093/annhyg/men081




