Diagnostic and Prognostic Biomarkers for Graft-Versus-Host Disease After Allogeneic Hematopoietic Stem Cell Transplantation
Problem Identification: A lack of testing options for the diagnosis and prognosis of chronic graft-versus-host disease (cGVHD) is a barrier to clinical management. Studies that have investigated the role of blood proteins as diagnostic and prognostic biomarkers for cGVHD were reviewed.
Literature Search: PubMed and Scopus databases were searched for articles published from January 1, 2000, to May 31, 2019. 660 articles were retrieved.
Data Evaluation: The authors appraised seven articles based on the inclusion and exclusion criteria to summarize identified blood protein biomarkers for cGVHD.
Synthesis: Several blood proteins were identified as potential diagnostic and prognostic biomarkers. Most of these proteins are thought to be key contributors in cGVHD pathogenesis and, therefore, could be ideal biomarkers to guide clinical management.
Implications for Practice: These biomarkers could aid providers in diagnosing cGVHD, identifying patients at high risk for development of cGVHD, and initiating preemptive therapy.
Jump to a section
Administration of donor stem cells, called allogeneic hematopoietic stem cell transplantation (allo-HSCT), is an effective therapeutic option for several hematologic malignancies. Allo-HSCT allows a patient to receive higher doses of chemotherapy and induce graft-versus-tumor effect for maximum tumor response. However, about 30%–70% of recipients after allo-HSCT will develop graft-versus-host disease (GVHD) (Zeiser & Blazar, 2017). The sequela of acute GVHD (aGVHD), chronic GVHD (cGVHD), or both after allo-HSCT can be a major cause of morbidity and mortality despite the use of immune-suppressive prophylaxis. Although the pathologic mechanisms are not clearly understood, the donor stem cells trigger an immunologic attack on single or multiple recipient organs, which can result in inflammation, decreased immunity, and fibrosis (Zeiser & Blazar, 2017). Depending on the severity of GVHD, the undesirable consequences can appear in the skin, gastrointestinal tract, liver, lungs, eyes, and genitals, and may cause functional and activity impairments, adverse general health, non-relapse mortality, dysfunctional organs, secondary malignancies, and poor quality of life (Wingard et al., 2011). Diagnosis of cGVHD can be particularly challenging because clinical manifestations may not present for as long as a year, and symptoms resemble other diseases, such as SjÖgren’s syndrome, scleroderma, wasting syndrome, chronic immunodeficiency, bronchiolitis obliterans, and primary biliary cirrhosis (Flowers & Vogelsang, 2009).
In an effort to predict and accurately diagnose GVHD, the role of biomarkers has shown potential benefit. Advances in protein biomarker research are paving the way for new tools in tackling diagnostic and prognostic challenges of aGVHD and cGVHD (Paczesny, 2018). According to the National Institutes of Health (NIH) consensus and the North American European Consortium, a biomarker is “a circulating protein or a biomolecule which can be measured objectively as an indicator of normal biological or pathologic processes, or biological and clinical responses to a therapeutic intervention” (Wolff et al., 2018, p. 833). Advances in technology have accelerated identification of specific and sensitive biomarkers, including those relevant to GVHD. The development of protein biomarkers for aGVHD has been fast-tracked and is being validated in a clinical trial to predict outcomes of patients with aGVHD (Blood and Marrow Transplant Clinical Trials Network [BMTCTN] Protocol 1501) (Levine et al., 2015). Contrarily, the exploratory research for protein biomarkers of cGVHD has been sluggish for reasons that include lack of understanding of the heterogeneous pathophysiology of cGVHD, clinical and pathologic overlap with aGVHD, varied clinical manifestations of cGVHD, longer pathologic trajectory, and lack of a sufficient number of study participants and multicenter trials. Recognizing the need to address challenges associated with diagnosis, prognosis, and clinical management of cGVHD, the NIH developed and published consensus reports. These reports were intended to streamline efforts in improving clinical management and identifying research priorities for cGVHD, which include determination of biomarkers that can aid in diagnostic and prognostic evaluation (Filipovich et al., 2005; Jagasia et al., 2015; Paczesny et al., 2015; Schultz et al., 2006).
In this integrative review, the authors analyze the results of research studies that discovered and reexamined reported blood protein biomarkers followed by their validation for diagnosis and prognosis of cGVHD. The authors discuss clinical implications for healthcare providers, including advanced practice nurses.
Methods
A comprehensive literature search was undertaken using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Moher et al., 2009) to retrieve relevant research on the use of blood proteins as biomarkers for cGVHD diagnosis and prognosis. Two electronic databases, PubMed and Scopus, were searched with the assistance of a medical librarian. The search terms used were hematopoietic stem cell transplantation OR stem cell transplantation OR stem cell transplant AND chronic graft versus host disease OR chronic graft-versus-host disease OR cGVHD AND proteomics OR proteins AND biomarker OR biomarkers AND Blood OR Plasma OR Serum. 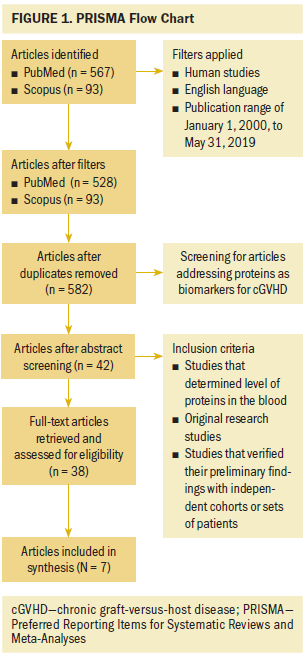
As shown in Figure 1, the initial search identified 567 articles from PubMed and 93 articles from Scopus. The respective search results were narrowed to 528 from PubMed and 93 from Scopus by applying the following inclusion criteria: human studies, English language, and publication dates from January 1, 2000, to May 31, 2019. The articles retrieved from both databases were then merged to remove duplicates, leading to a total of 582 articles. Titles and abstracts of these 582 articles were screened to identify articles that addressed blood proteins as biomarkers for cGVHD. Therefore, the search was narrowed to 42 articles. Of these, only 38 were full-text articles, which were screened further to identify studies that met the following inclusion and exclusion criteria. The inclusion criteria were (a) studies that determined the level of proteins in the blood, (b) studies that verified their preliminary findings with independent cohorts or sets of patients, and (c) original research studies in adult patients with hematologic malignancies. The exclusion criteria were (a) articles that reported aGVHD biomarkers, (b) articles with non-protein biomarkers, (c) articles with non–blood-based protein biomarkers, (d) articles that did not validate preliminary findings with an independent set of patients, (e) review articles, (f) case studies, (g) editorials, and (h) abstracts. Seven articles met all criteria for inclusion in this integrative review. The references of all of the selected articles were also screened for relevant articles, but no additional studies were identified. 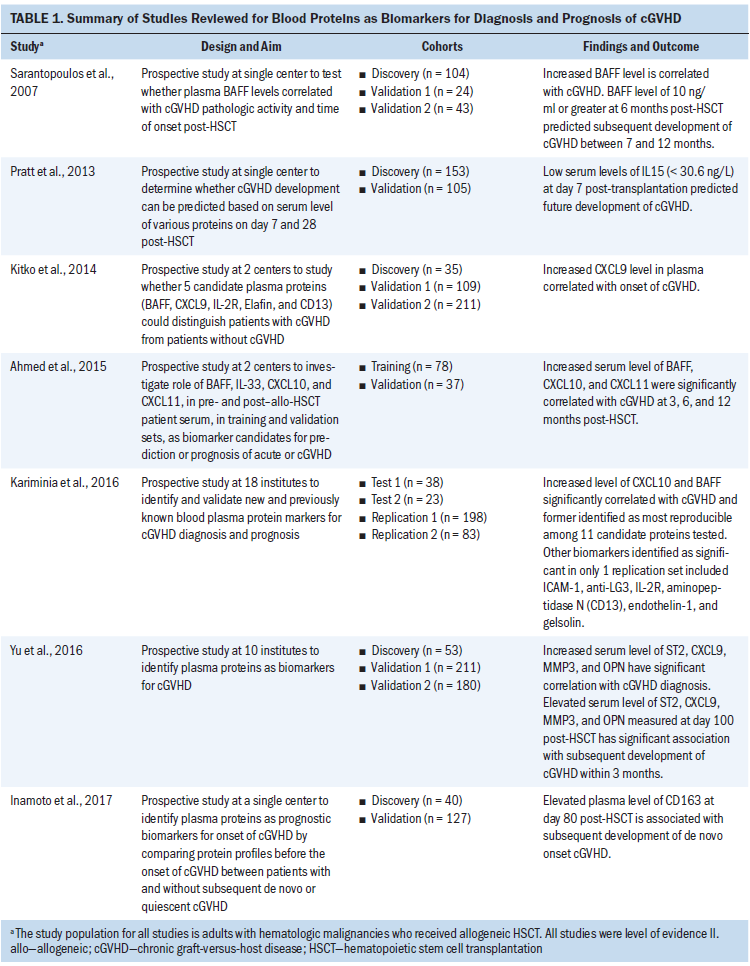
Results
The characteristics and findings of all selected articles are summarized in Table 1. The results of the studies are organized into two major sections: diagnostic biomarkers and prognostic biomarkers. A summary of results is presented in Table 2. 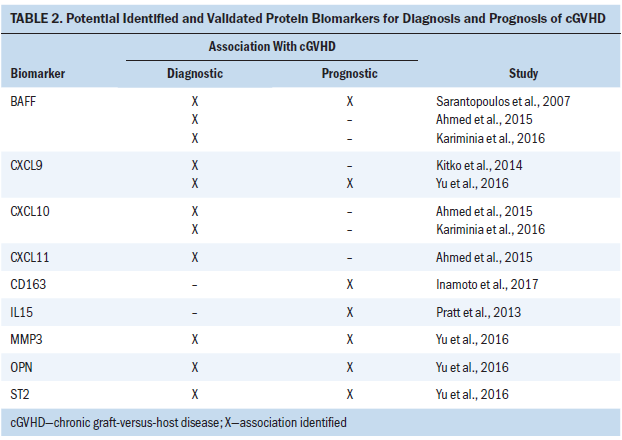
Diagnostic Biomarkers
Five studies reported the use of seven proteins (BAFF, CXCL9, CXCL10, CXCL11, ST2, MMP3, and OPN) as potential diagnostic biomarkers for cGVHD (Ahmed et al., 2015; Kariminia et al., 2016; Kitko et al., 2014; Sarantopoulos et al., 2007; Yu et al., 2016).
An initial study by Sarantopoulos et al. (2007) used independent cohorts to investigate the role of a single known protein, BAFF, and employed one discovery and two validation cohorts in a prospective study at a single center. The BAFF levels were significantly higher in all patients post-HSCT compared to healthy donor participants (p < 0.0001). Patients in the validation cohorts with serial BAFF levels of 10 ng/ml or greater developed cGVHD as compared to those whose levels decreased to less than 10 ng/ml. In addition, the BAFF levels were also significantly higher in patients with cGVHD compared to patients who never had or currently had inactive cGVHD (p = 0.0002). These results were suggestive of BAFF’s potential as a diagnostic biomarker for cGVHD.
Kitko et al. (2014) attempted to identify multiple protein biomarkers, including BAFF for newly diagnosed or de novo cGVHD. Using microarray technique, five proteins (CXCL9, Elafin, IL2Ra, CD13, and BAFF) were identified, which could distinguish patients with cGVHD from those without cGVHD (p < 0.1). In validation cohort 1 (n = 109), a significant increase was noted in plasma levels of all five proteins in the de novo cGVHD group compared to the no cGVHD group, confirming the results of the discovery cohort. The chi-square analysis also revealed strong correlation between CXCL9 plasma concentrations and presence of de novo onset cGVHD (p < 0.001). Additional examination of CXCL9 in validation cohort 2 demonstrated significant elevation of CXCL9 plasma levels in de novo onset cGVHD compared to non-cGVHD (p = 0.003, area under the curve [AUC] = 0.68). The researchers concluded that greater than 6.5 pg/ml of plasma concentration of CXCL9 could be a diagnostic biomarker for de novo onset cGVHD.
Ahmed et al. (2015) also tested four inflammatory serum proteins (BAFF, IL-33, CXCL10, and CXCL11) that were selected based on previously published reports. Collectively, elevated levels of BAFF, CXCL10, and CXCL11 suggested a significant association with cGVHD at 3, 6, and 12 months. The researchers combined training and validation cohort results to evaluate the clinical potential of each of these proteins as a diagnostic biomarker for cGVHD and concluded that BAFF, CXCL10, and CXCL11 could be useful.
Although these studies were carried out either at a single center or two centers, a study spanning 18 institutions across the United States, Canada, Germany, and Saudi Arabia by Kariminia et al. (2016) investigated 11 diagnostic biomarkers for cGVHD in four independent sets of patients. Only CXCL10 met the criterion to be of highest interest for diagnostic use.
Unlike the previous studies, Yu et al. (2016) employed a different methodologic approach by pooling plasma samples of patients for identification and quantification of proteins that could distinguish cGVHD from non-cGVHD. In validation cohort 1, four proteins (ST2, MMP3, CXCL9, and OPN) were found to be significant (p < 0.001, AUC = 0.92). These four proteins were further verified in validation cohort 2 (p < 0.001, AUC = 0.82). Based on the findings, the study authors concluded that these four proteins had a significant correlation with cGVHD and could be potential diagnostic biomarkers.
Prognostic Biomarkers
Four studies determined the use of seven blood proteins (CD163, IL15, BAFF, CXCL9, ST2, MMP3, and OPN) as prognostic biomarkers for cGVHD (Inamoto et al., 2017; Pratt et al., 2013; Sarantopoulos et al., 2007; Yu et al., 2016). Based on the significant association of BAFF (10 ng/ml or greater) with cGVHD, Sarantopoulos et al. (2007) also examined its prognostic role. In validation cohort 2, 77% of the patients with BAFF levels of 10 ng/ml or greater compared to 12% of the patients with BAFF levels of less than 10 ng/ml subsequently developed cGVHD (p < 0.0001). These findings were indicative of BAFF as a potential prognostic biomarker for cGVHD. Similarly, the four proteins (ST2, MMP3, CXCL9, and OPN) identified by Yu et al. (2016) also predicted future cGVHD development within three months (p = 0.009; AUC = 0.67). These findings suggest that multiple proteins could serve as a potential prognostic biomarker panel for cGVHD.
cGVHD usually develops about 100 days post-HSCT (Zeiser & Blazar, 2017). However, Pratt et al. (2013) collected blood samples at approximately day 7 and day 28 and investigated 13 selected proteins (BAFF, VEGF, TNF-aR1, IL-2Ra, IL5, IL6, IL7, IL15, gamma-GTP, cholinesterase, total protein, urea, and ATG) to see if they predicted future cGVHD development. In a discovery cohort, univariate analysis followed by multivariate analysis revealed low serum levels of IL15 (less than 30.6 ng/L) at day 7 post-HSCT had a 2.7-fold higher risk for developing cGVHD (p < 0.001). The validation cohort analysis revealed the same trend with 3.7-fold higher likelihood of cGVHD development with low IL15 serum levels at day 7 post-HSCT (p = 0.001). This study alone identified decreased rather than increased levels of blood proteins as potential prognostic cGVHD biomarkers.
In a study to identify prognostic biomarkers for de novo or quiescent onset cGVHD, Inamoto et al. (2017) enrolled 167 consecutive patients without any signs of clinical cGVHD manifestations. In a discovery cohort, the researchers found 20 of 400 proteins (SCAND3, KCNH, KIAA1958, IGHM, HPR, LGALS3BP, GIP, CD5L, LTBP2, SH3BGRL3, LILRA3, CD163, GUCA2A, CRTAC1, PROZ, PRDX6, TFF2, TXN, HSP90B1, and PDLIM1) with concentrations either 1.5-fold higher or 0.66-fold lower than the control group. The researchers eventually identified four proteins (LGALS3BP, CD5L, CD163, and TXN) for additional verification in the validation cohort with 127 patients. The results of the validation cohort revealed a significant correlation between higher plasma concentration of CD163 at day 80 post-HSCT, with a higher cumulative incidence of subsequent development of de novo cGVHD (p = 0.018).
Findings
The results from the selected articles revealed that BAFF, CXCL9, CXCL10, CXCL11, ST2, MMP3, and OPN could be useful as diagnostic biomarkers, and CD163, IL15, BAFF, CXCL9, ST2, MMP3, and OPN could be potential prognostic biomarkers. With established cGVHD assessment guidelines, blood samples for analyses of potential biomarkers could be collected at any time post-HSCT to confirm cGVHD phenotype. To predict future development of cGVHD, blood samples could be collected as early as day 7 post-HSCT. Some of these studies also had other aims, such as association of biomarker levels with severity or grade of cGVHD, specific organ involvement, non-relapse mortality, or systemic steroid treatment response. Because these aims were beyond the scope of this integrative review, the results were not incorporated or analyzed.
Discussion
Most of the proteins verified by these studies were previously known or suspected to play a crucial role in cGVHD pathogenesis, which make them logical candidates for diagnostic or prognositic indicators. For example, three selected studies reported a correlation of elevated BAFF levels with cGVHD, which could be diagnostic (Ahmed et al., 2015; Kariminia et al., 2016; Sarantopoulos et al., 2007). Sarantopoulos et al. (2007) also reported sustained elevated levels of BAFF at six months post-HSCT, which could be predictive for subsequent cGVHD. BAFF is a cytokine belonging to the tumor necrosis ligand family of proteins and affects B-cell survival and maturation at multiple levels and, therefore, takes part in the inflammation and regulation of immune responses. The increased levels of BAFF in blood reported by these studies are indicative of increased cGVHD activity and could possibly play a role in underlying inflammatory, as well as immune, reactions of cGVHD pathogenesis. Therefore, high levels of BAFF in the blood may be used to corroborate clinical findings of cGVHD. In addition, as seen in the study by Sarantopoulos et al. (2007), sustained elevation of BAFF in post-HSCT settings could be an indicator to initiate preemptive therapy to prevent subsequent cGVHD development. These findings suggest that elevated BAFF levels may render this biomarker as both a prognostic and a diagnostic tool in the management of patients undergoing HSCT.
Contrary to the elevated blood levels of BAFF, low levels of another cytokine, IL15, at day 7 post-HSCT could predict future cGVHD development (Pratt et al., 2013). IL15 has been reported as a stimulator for CD8-positive T-cell proliferation, cytotoxic T-lymphocyte induction facilitator, as well as NK-cell generator and activator. Pratt et al. (2013) found a negative correlation between IL15 levels and CD8 T-cell counts. These results imply that low levels of IL15 may be a key player in cGVHD pathogenesis through acting as a surrogate for proliferation and survival of CD8 T cells (Pratt et al., 2013).
Many other protein biomarkers, such as CXCL9, CXCL10, CXCL11, and ST2, verified by these selected studies, work collectively upstream or downstream of each other in the inflammatory process. Three chemokines (CXCL9, CXCL10, and CXCL11), when elevated in the blood, correlated with the presence of cGVHD, which may indicate their diagnostic potential. In addition, in one study, CXCL9 was found to be predictive for future cGVHD pathogenesis. These chemokines are ligands that bind only to their receptor, CXCR3. Together, the CXCR3-CXCL9/10/11 complex constitutes an important pathologic pathway to recruit effector cells in the post-HSCT environment (Croudace et al., 2012). Of note, the CXCR3-CXCL9/10/11 complex is a downstream pathway to another biomarker, ST2. Increased expression of ST2 evokes a danger signal apparent to immune cells. This then initiates an inflammatory response and alerts CXCR3-CXCL9/10/11 complex. This fact aligns with the elevated levels of CXCL9 and ST2 found by Yu et al. (2016) and is in support of the diagnostic and prognostic potential of these two proteins.
Another integral part of inflammation is monocyte and macrophage activation, which could contribute to subsequent development of cGVHD (Inamoto et al., 2017). Inflammatory cytokines, such as IL6 and IL10, are known to increase expression of CD163 receptor sites, which are located on monocyte and macrophage cells, leading to their activation. Increased plasma concentration of CD163, reported by Inamoto et al. (2017), is reflective of its pathogenic role and, therefore, its potential as a biomarker.
MMP3 is a protein known for degrading the basement and extracellular membrane to facilitate pathologic changes, such as cell migration, infiltration, and tissue remodeling (O’Sullivan et al., 2015). In addition, elevated levels of MMP3 have been reported in patients with inflammatory conditions and associated with cellular damage (Kobayashi et al., 2007). Increased levels of MMP3 observed by Inamoto et al. (2017) could be a sign of structural damage in organs afflicted with cGVHD. Therefore, most of the verified proteins appear to be crucial players in underlying pathogenesis of cGVHD and could be ideal biomarkers for cGVHD.
The major inconsistencies among these selected studies are single-center versus multicenter studies, varying sample collection times, cGVHD participants with or without a prior history of aGVHD, use of different protein detection methods (enzyme-linked immunosorbent assay [ELISA], fluorescence activated cell sorting [FACS] analysis, mass spectrometric analysis, and antibody array), study participants on steroid treatment, and patients with different HSCT treatment modalities. These limitations hinder the generalizability of the results. However, despite these inconsistencies, study design was a major strength of the articles reviewed. The discovery–validation cohort study design makes the association of biomarkers with cGVHD well substantiated. The other major strength is the level of evidence, with all studies being level II.
The selected articles reflect the impact of the NIH consensus recommendations, depending on timing of the study before or after the NIH publication in 2014. For example, two of the NIH recommendations included multiple rather than a single protein to adjust for diverse pathophysiologic pathways and a sufficient number of patients within multicenter trials to adjust for the heterogeneity of patients with cGVHD. Accordingly, Sarantopoulos et al. (2007) published their study, which examined a single known protein, BAFF, in a prospective study at a single center. However, Kariminia et al. (2016) published their study that explored unidentified blood proteins in two separate cohorts of patients leading to the identification of multiple (seven) proteins. These authors also added four known proteins either previously known to have a link with cGVHD or presumably known to have pathologic relevance with cGVHD. Therefore, 11 proteins were qualified for further verification in two independent replication sets. It is clearly evident from these two studies (one published before and one after NIH consensus in 2014) that larger cohorts at multiple centers to explore and validate multiple proteins while using uniform protein detection methods would be a better option in the quest to identify protein biomarkers associated with cGVHD.
Implications for Practice
The use of protein biomarkers has the potential to offer diagnostic and prognostic value for clinicians, including nursing professionals providing care to patients undergoing allo-HSCT. Clinical use of the protein biomarkers discussed is limited at this time because validity is still being investigated. In the future, it will be within the nurse practitioner’s scope of practice to order and interpret protein biomarker results that can aid in determining diagnosis, prognosis, disease severity, treatment plan, therapy response, and end-of-life considerations. Nurses in clinical practice could also use knowledge of protein biomarker testing to educate patients about the importance of such testing, explain the meaning of results, and formulate an appropriate nursing plan of care. Research nurses could help in enrolling patients in clinical trials or research studies and educating patients in how their participation could help advance clinical practice and patient outcomes. Nurse researchers could contribute by undertaking studies to see if blood proteins could predict cGVHD symptom burden by exploring possible association between levels of proteins and severity of cGVHD symptoms. Finally, nurse educators could equip the next generation of nurses, nurse practitioners, and other nursing professionals with the knowledge of protein biomarker science and its clinical applicability. 
Conclusion
In the analysis of the seven peer-reviewed articles selected for this integrative review, five salient features emerged:
• BAFF, CXCL9, CXCL10, CXCL11, ST2, MMP3, and OPN are potential diagnostic protein biomarkers for cGVHD.
• CD163, IL15, BAFF, CXCL9, ST2, MMP3, and OPN are potential prognostic protein biomarkers for cGVHD.
• With established cGVHD assessment guidelines, blood samples for analyses of potential biomarkers could be collected at any time post-HSCT to confirm cGVHD phenotype. To predict future development of cGVHD, blood samples could be collected as early as day 7 post-HSCT.
• Given the complexity of the disease process, a panel of multiple proteins or a combination of proteins could be the best approach in future biomarker studies.
• It may be prudent to expand the search for cGVHD biomarkers based on NIH consensus recommendations. Multicenter, large-scale investigational approaches could help identify additional proteins, as well as unravel unknown cGVHD pathogenic pathways.
Discovery and validation of biomarkers with good sensitivity and specificity are also warranted. Because of the complexity of cGVHD pathology, as well as multiple organ involvement, it is crucial to identify organ-specific biomarkers. Corticosteroids have remained a nonspecific mainline therapy for cGVHD and, therefore, efforts should be directed to identify drug-targetable biomarkers.
The authors gratefully acknowledge Laurissa Gann, MSLS, AHIP, medical librarian at MD Anderson Cancer Center, for her assistance with the literature search.
About the Author(s)
Nilesh Kalariya, PhD, APRN, AGPCNP-BC, AOCNP®, is an APRN oncology fellow, Christi Bowe, MSN, APRN, ANP-C, is the associate director of advanced practice nursing, and Joyce E. Dains, DrPH, JD, APRN, FNP-BC, FNAP, FAANP, is a professor, chair ad interim, and director of advanced practice nursing, all in the Department of Nursing at the University of Texas MD Anderson Cancer Center in Houston. No relevant financial relationships to disclose. All authors contributed to the conceptualization and design and manuscript preparation. Kalariya completed the data collection and provided statistical support. Kalariya and Dains provided analysis. Kalariya can be reached at nmkalariya@mdanderson.org, with copy to ONFEditor@ons.org. (Submitted June 2019. Accepted November 4, 2019.)
References
Ahmed, S.S., Wang, X.N., Norden, J., Pearce, K., El-Gezawy, E., Atarod, S., . . . Dickinson, A.M. (2015). Identification and validation of biomarkers associated with acute and chronic graft versus host disease. Bone Marrow Transplantation, 50(12), 1563–1571. https://doi.org/10.1038/bmt.2015.191
Croudace, J.E., Inman, C.F., Abbotts, B.E., Nagra, S., Nunnick, J., Mahendra, P., . . . Moss, P.A.H. (2012). Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T cells plays a major role in the pathogenesis of chronic GVHD. Blood, 120(20), 4246–4255. https://doi.org/10.1182/blood-2012-02-413260
Filipovich, A.H., Weisdorf, D., Pavletic, S., Socie, G., Wingard, J.R., Lee, S.J., . . . Flowers, M.E. (2005). National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of Blood and Marrow Transplantation, 11(12), 945–956.
Flowers, M.E.D., & Vogelsang, G.B. (2009). Clinical manifestations and natural history. In G.B. Vogelsang & S.Z. Pavletic (Eds.), Chronic graft versus host disease: Interdisciplinary management (pp. 56–69). Cambridge University Press.
Inamoto, Y., Martin, P.J., Paczesny, S., Tabellini, L., Momin, A.A., Mumaw, C.L., . . . Hansen, J.A. (2017). Association of plasma CD163 concentration with de novo-onset chronic graft-versus-host disease. Biology of Blood and Marrow Transplantation, 23(8), 1250–1256. https://doi.org/10.1016/j.bbmt.2017.04.019
Jagasia, M.H., Greinix, H.T., Arora, M., Williams, K.M., Wolff, D., Cowen, E.W., . . . Flowers, M.E.D. (2015). National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biology of Blood Marrow Transplantation, 21(3), 389–401. https://doi.org/10.1016/j.bbmt.2014.12.001
Kariminia, A., Holtan, S.G., Ivison, S., Rozmus, J., Hebert, M.J., Martin, P.J., . . . Schultz, K.R. (2016). Heterogeneity of chronic graft-versus-host disease biomarkers: Association with CXCL10 and CXCR3+ NK cells. Blood, 127(24), 3082–3091. https://doi.org/10.1182/blood-2015-09-668251
Kitko, C.L., Levine, J.E., Storer, B.E., Chai, X., Fox, D.A., Braun, T.M., . . . Paczesny, S. (2014). Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood, 123(5), 786–793. https://doi.org/10.1182/blood-2013-08-520072
Kobayashi, A., Naito, S., Enomoto, H., Shiomoi, T., Kimura, T., Obata, K., . . . Okada, Y. (2007). Serum levels of matrix metalloproteinase 3 (stromelysin 1) for monitoring synovitis in rheumatoid arthritis. Archives of Pathology and Laboratory Medicine, 131, 563–570. https://doi.org/10.1043/1543-2165(2007)131[563:SLOMMS]2.0.CO;2
Levine, J.E., Braun, T.M., Harris, A.C., Holler, E., Taylor, A., Miller, H., . . . Ferrara, J.L.M. (2015). A prognostic score for acute graft-versus-host disease based on biomarkers: A multicentre study. Lancet. Haematololgy, 2(1), e21–e29. https://doi.org/10.1016/S2352-3026(14)00035-0
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed1000097
O’Sullivan, S., Gilmer, J.F., & Medina, C. (2015). Matrix metalloproteinases in inflammatory bowel disease: An update. Mediators of Inflammation, 2015, 964131. https://doi.org/10.1155/2015/964131
Paczesny, S. (2018). Biomarkers for posttransplantation outcomes. Blood, 131(20), 2193–2204. https://doi.org/10.1182/blood-2018-02-791509
Paczesny, S., Hakim, F.T., Pidala, J., Cooke, K.R., Lathrop, J., Griffith, L.M., . . . Schultz, K.R. (2015). National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. The 2014 Biomarker Working Group Report. Biology of Blood and Marrow Transplantation, 21(5), 780–792. https://doi.org/10.1016/j.bbmt.2015.01.003
Pratt, L.M., Liu, Y., Ugarte-Torres, A., Hoegh-Petersen, M., Podgorny, P.J., Lyon, A.W., . . . Storek, J. (2013). IL15 levels on day 7 after hematopoietic cell transplantation predict chronic GVHD. Bone Marrow Transplantion, 48(5), 722–728. https://doi.org/10.1038/bmt.2012.210
Sarantopoulos, S., Stevenson, K.E., Kim, H.T., Bhuiya, N.S., Cutler, C.S., Soiffer, R.J., . . . Ritz, J. (2007). High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clinical Cancer Research, 13(20), 6107–6114. https://doi.org/10.1158/1078-0432.ccr-07-1290
Schultz, K.R., Miklos, D.B., Fowler, D., Cooke, K., Shizuru, J., Zorn, E., . . . Pavletic, S.Z. (2006). Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biology of Blood and Marrow Transplantation, 12(2), 126–137. https://doi.org/10.1016/j.bbmt.2005.11.010
Wingard, J.R., Majhail, N.S., Brazauskas, R., Wang, Z., Sobocinski, K.A., Jacobsohn, D., . . . Socié G. (2011). Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Journal of Clinical Oncology, 29(16), 2230–2239.
Wolff, D., Greinix, H., Lee, S.J., Gooley, T., Paczesny, S., Pavletic, S., . . . Schultz, K.R. (2018). Biomarkers in chronic graft-versus-host disease: Quo vadis? Bone Marrow Transplantation, 53(7), 832–837. https://doi.org/10.1038/s41409-018-0092-x
Yu, J., Storer, B.E., Kushekhar, K., Abu Zaid, M., Zhang, Q., Gafken, P.R., . . . Paczesny, S. (2016). Biomarker panel for chronic graft-versus-host disease. Journal of Clinical Oncology, 34(22), 2583–2590. https://doi.org/10.1200/JCO.2015.65.9615
Zeiser, R., & Blazar, B.R. (2017). Pathophysiology of chronic graft-versus-host disease and therapeutic targets. New England Journal of Medicine, 377(26), 2565–2579.




