Barriers and Facilitators to Cancer Screening Among LGBTQ Individuals With Cancer
Problem Identification: Cancer screening may reduce mortality and frequency of the disease. Lesbian, gay, bisexual, transgender, or queer (LGBTQ) individuals are less likely than non-LGBTQ individuals to present for cancer screening.
Literature Search: A literature search was performed using CINAHL®, PsycINFO®, and PubMed®. Articles were included if they were published in English from 2008 to 2018 and addressed barriers or facilitators to cancer screening in LGBTQ populations.
Data Evaluation: Data were organized by thematic matrix and classified according to the multilevel influences on the cancer care continuum framework: individual patient, family and social supports, provider/team, organization and/or practice setting, local community environment, state health policy environment, and national health policy environment.
Synthesis: This integrative review found that the lack of cancer screening data and knowledge about screening guidelines by LGBTQ populations and providers were major barriers to cancer screening adherence. Provider-created welcoming environments and caregiver inclusion were facilitators.
Implications for Practice: Determinants of health-seeking behavior included patients’ and providers’ lack of cancer screening knowledge, as well as perceived discrimination. Nurses are in a unique position to provide cancer screening information and culturally sensitive care for LGBTQ populations with adequate education.
Jump to a section
According to 2017 Gallup poll results, 11 million Americans, or 4.5% of the U.S. population, identify as lesbian, gay, bisexual, transgender, or queer (LGBTQ); this is an increase from 3.5% in 2012, when Gallup began tracking LGBTQ populations-based data (Newport, 2018). Despite this increase, the Centers for Disease Control and Prevention’s National Program of Cancer Registries, the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, and the American College of Surgeons and the American Cancer Society’s National Cancer Data Base do not collect sexual orientation or gender identification (SOGI) data in regard to cancer surveillance. Therefore, limited cancer screening data exist for LGBTQ populations.
Research has shown that LGBTQ populations have the highest rates of tobacco and alcohol use, both of which are known contributors to elevated cancer risk, compared to non-LGBTQ populations (Daniel & Butkus, 2015). These elevated risks lead to a disproportionate number of LGBTQ individuals living with cancers, including anal, breast, cervical, colorectal, endometrial, lung, and prostate (Bristowe et al., 2018; Burkhalter et al., 2016; Gonzales & Zinone, 2018; Gruskin, Hart, Gordon, & Ackerson, 2001; Institute of Medicine, 2011; McCabe, West, Hughes, & Boyd, 2013; McComiskey et al., 2018; Tang et al., 2004). However, reasons for participation in cancer screening are not largely documented and can only be speculative without collection of cancer-specific SOGI data (Burkhalter et al., 2016; Quinn et al., 2015). Therefore, a literature review was conducted to provide a better understanding of the barriers and facilitators to LGBTQ populations’ cancer screening behavior.
The integrative review framework by Whittemore and Knafl (2005) guided this review and was implemented using the following steps: problem identification, literature search, data evaluation, data analysis, and presentation. Current literature was explored using the multilevel influences on the cancer care continuum (MICCC) framework (Taplin et al., 2012) through the lens of queer theory. The MICCC, an adapted social ecological model, was used to categorize barriers and facilitators identified during the literature review (Taplin et al., 2012). This framework posits that health behavior is a product of seven levels of influence: individual patient, family and social supports, provider/team, organization and/or practice setting, local community environment, state health policy environment, and national health policy environment. An individual’s experience with the healthcare system is complex and influenced between and across levels. Changes at single levels may not have a trickle-down effect, so health behavior changes should be targeted at multiple levels (Taplin et al., 2012).
Queer theory suggests that the binary nature of heteronormativity, through institutional practices, creates systematic discrimination against those who do not conform to traditional gender roles. Heteronormativity refers to “the assumption that heterosexuality is the default, preferred, ‘normal’ state for human beings due to the belief that people fall into one category of a strict gender binary” (Harris & White, 2018, p. 249). These assumptions create systems ostracizing individuals whose SOGI status is nonheteronormative or gender nonconforming (Giffney, 2004) and lead to “prejudiced attitudes, mistreatment and discriminatory practices that are directed at people who are disadvantaged by imbalances of power in society on the basis of their sexuality” (Harris & White, 2018, p. 241). First coined by Italian sociologist Annmarie Jagose (1997), queer theory has its roots in writings by scholars Michel Foucault (1995), Eve Kosofsky Sedgwick (2005), Lauren Berlant (2006), Leo Bersani (Tuhkanen, 2014), Judith Butler (2008), Lee Edelman (2004), and Jack Halberstam (2005).
This review will examine ways that heteronormativity influences cancer screening participation and providers’ treatment of LGBTQ individuals; factors on various levels of the MICCC framework within the context of queer theory and their cumulative effect on individual cancer screening behavior; barriers that lend to the determinants of health-seeking behavior; and facilitators in adherence to cancer screening in LGBTQ populations (Stokols, 1996; Taplin et al., 2012).
Methods
CINAHL®, PsycINFO®, and PubMed® were searched to identify relevant articles for inclusion. The following search terms were used: lgbt OR gay OR homosexual OR lesbian OR bisexual OR transgender OR queer OR questioning OR men who have sex with men OR msm OR high risk behaviors AND cancer screening. Articles were included if they were data-based research studies of cancer screening barriers and facilitators in LGBTQ populations, published from 2008 to 2018, written in English, and conducted in the United States. Articles were excluded if they focused predominantly on HIV because additional layers of barrier/facilitator influence are attributed to HIV that are outside the realm of this review. The literature search was limited to articles published from 2008 onward because of changes in the legal landscape since then, as well as an increase in acceptance of LGBTQ populations; the articles selected for inclusion, then, were representative of the current cultural environment (Brown, 2017).
The initial electronic database search produced a total of 150 publications; of these, 78 potentially relevant abstracts were reviewed, 54 abstracts were excluded, 24 full-text articles were retrieved, and 11 articles met inclusion criteria. One identified article described the qualitative results of a larger mixed methods study, so the parent study was included instead. In addition, a bibliographic hand search that was performed identified one additional article for a final sample size of 12.
The 12 articles were critically examined, and a matrix was created to document each study’s methodology, sample and characteristics, themes, design, and outcomes. Table 1 presents characteristics of the reviewed studies. Additional thematic tables outlining common barriers and facilitators identified in the studies were created. 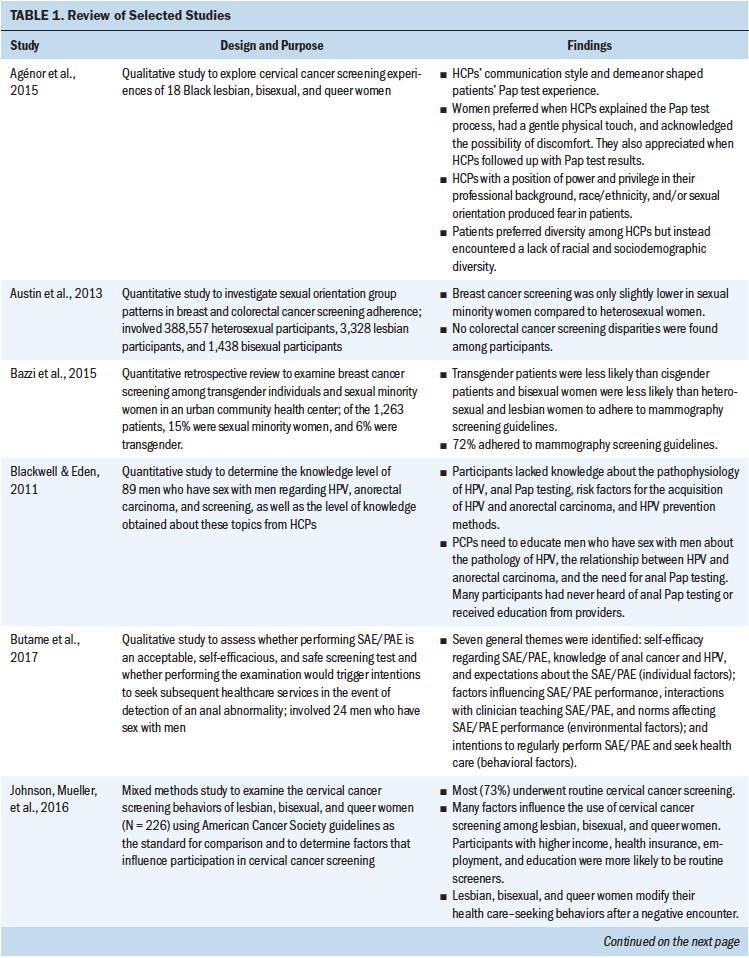
Results
The resulting themes were classified into one or more of the eight MICCC framework levels of influence. Queer theory was used to highlight the privilege, unequal power distribution, and oppression that heteronormativity creates (Giffney, 2004) and the feelings of marginalization in LGBTQ populations (Worthen, 2016).
Sample Characteristics
The majority of studies (n = 8) used a quantitative design with survey or questionnaire administration (Austin et al., 2013; Bazzi, Whorms, King, & Potter, 2015; Blackwell & Eden, 2011; Polek & Hardie, 2010; Reed, Reiter, Smith, Palefsky, & Brewer, 2010; Tabaac, Sutter, Wall, & Baker, 2018; Thompson, Reiter, McRee, Moss, & Brewer, 2015; Tracy, Lydecker, & Ireland, 2010). Three studies used a qualitative design (Agénor, Bailey, Krieger, Austin, & Gottlieb, 2015; Butame et al., 2017; Peitzmeier et al., 2017), and one study used a mixed methods design (Johnson, Mueller, Eliason, Stuart, & Nemeth, 2016).
Barriers to Cancer Screening Activity
Individual patient: In the studies selected for review, gender identity and expression, racial and ethnic identity, socioeconomic status, provider and patient knowledge gaps, poor psychosocial reactions and emotional coping, and lower educational attainment were identified as individual patient barriers to screening activity. Gender identity, gender expression, and sexual orientation were found to be biological factors that acted as barriers to cancer screening in the reviewed studies. Being gender nonconforming (i.e., not conforming to one particular gender [Human Rights Campaign, n.d.]), transgender, or bisexual correlated with lower likelihood of presentation for screening for breast, cervical, and colon cancer (Bazzi et al., 2015; Johnson, Mueller, et al., 2016; Tabaac et al., 2018).
Racial and ethnic identity influenced individuals’ perceptions of provider discrimination (Agénor et al., 2015; Tracy et al., 2010). Minority status led to overall lower rates of adherence to cervical cancer screening for lesbian, gay, bisexual, and transgender individuals (Tracy et al., 2010). Black lesbian, bisexual, and queer women reported negative screening experiences in which they perceived discrimination from their providers (Agénor et al., 2015).
When lesbian or bisexual women had less educational attainment, they were more likely to be nonadherent to cervical cancer screening (Johnson, Mueller, et al., 2016; Tracy et al., 2010). Compared to lesbian women with less than or only a high school education, lesbian women with a college education but not a graduate degree were less likely to know of the connection between human papillomavirus (HPV) and cancer (Polek & Hardie, 2010). However, lesbian women with less than a college education were better informed about the HPV–cancer connection than those with a college degree but did not differ from those with a graduate degree (Polek & Hardie, 2010). Financial instability was found to be a barrier to mammography and cervical cancer screening (Bazzi et al., 2015; Johnson, Mueller, et al., 2016).
Lack of knowledge about LGBTQ-specific cancer screening guidelines in LGBTQ populations and among providers contributed to poor screening activity (Blackwell & Eden, 2011; Butame et al., 2017; Peitzmeier et al., 2017; Polek & Hardie, 2010; Reed et al., 2010; Thompson et al., 2015; Tracy et al., 2010). In addition, lack of knowledge about HPV’s ability to be transmitted through anal sex or female-to-female contact and its link to the development of squamous cell carcinoma was found in gay men and lesbian women (Butame et al., 2017; Polek & Hardie, 2010). Of note, women who were open about their sexual orientation with providers were less likely to know about HPV spread through female-to-female contact (Polek & Hardie, 2010). This lack of knowledge intersects with provider- and team-level barriers, suggesting that the women who informed their provider of their sexual orientation were not told about the risks of HPV transmission, although reasons for this are unclear from the reviewed study (Polek & Hardie, 2010).
Psychosocial and coping-related factors, primarily gender incongruence and psychological distress, negatively affected transgender female to male individuals (i.e., transgender men) when presenting for a Papanicolaou (Pap) test to screen for cervical cancer (Peitzmeier et al., 2017). In addition, they described experiencing severe emotional distress when presenting for a Pap test, as it is a procedure involving female anatomy, which does not align with their identified gender (Peitzmeier et al., 2017). When transgender men perceived fewer benefits of screening for cervical cancer and believed they were less susceptible to cervical cancer, they were less likely to present for screening (Tabaac et al., 2018). Accordingly, gender dysphoria and identity destabilization were identified as pertinent risks to pursuing cervical cancer screening in transgender women (Tabaac et al., 2018). Gender dysphoria refers to the existence of conflict between an individual’s physical or assigned gender and the gender with which they identify (Parekh, 2016). Identity destabilization occurs when an individual’s idea of who they are is threatened (Tracy et al., 2010).
Family and social supports: Although not statistically significant, participants in the Johnson, Mueller, et al. (2016) study described a lack of peer support and role models as having a negative impact on cervical cancer screening activity among lesbian, bisexual, and queer women, as well as transgender men.
Provider/team: Healthcare providers and members of the healthcare team affect LGBTQ populations’ care delivery in many ways. Themes that arose on the provider/team level as barriers to screening were knowledge and communication, cultural competency, and teamwork. Lack of knowledge and poor communication skills in providers were strong barriers to screening in many of the reviewed studies. When healthcare providers assumed that the patients’ SOGI aligned with their sex assigned at birth, appropriate cancer screening recommendations did not occur (Agénor et al., 2015; Polek & Hardie, 2010). It is theorized that this is because of lack of patient comfort in disclosure and perceived discrimination (Agénor et al., 2015; Blackwell & Eden, 2011; Johnson, Mueller, et al., 2016; Polek & Hardie, 2010; Tabaac et al., 2018). When discrimination based on gender identity was reported by transgender men, they were as much as 3.3 times less likely to have routine cervical cancer screening (Johnson, Mueller, et al., 2016). Lower rates of Pap tests among transgender men, colorectal cancer screening among transgender women, and prostate-specific antigen tests for prostate cancer among gender-nonconforming individuals were reported (Tabaac et al., 2018). In addition, providers lacked knowledge that the anal Pap test could be used for HPV screening and that the test was too expensive to be afforded by some individuals (Reed et al., 2010). Providers with higher percentages of female patients had a higher likelihood of mammography screening (Bazzi et al., 2015).
Perceived lack of cultural competency by the provider also limited lesbian, bisexual, and transgender populations’ comfort with obtaining the appropriate screening (Polek & Hardie, 2010). Transgender male patients experienced the need to negotiate gender with their provider when presenting for cervical cancer screening; this existed in the form of teaching the provider about gender status because of inappropriate, excessive, or invasive questions about sexual practices or anatomical surgeries (Peitzmeier et al., 2017). It was also noted that when transgender patients had to negotiate for their own needs to not feel dehumanized or deindividualized, they developed negative views regarding screening, which prevented future screening (Peitzmeier et al., 2017).
When LGBTQ patients did not feel a sense of teamwork with their providers, a lack of trust existed and was associated with negative experiences when presenting for cervical cancer screening (Agénor et al., 2015; Peitzmeier et al., 2017). As previously noted, a negative relationship between openness with a provider and knowledge of female-to-female HPV existed in patients and providers (Polek & Hardie, 2010). Therefore, even when disclosure was established between the provider and the LGBTQ patient, providers were not necessarily knowledgeable about screening practices or recommended them (Agénor et al., 2015; Peitzmeier et al., 2017; Polek & Hardie, 2010). Fear of discrimination from providers was the most frequently reported barrier to obtaining and presenting for cancer screening among LGBTQ populations (Agénor et al., 2015; Peitzmeier et al., 2017; Polek & Hardie, 2010; Tracy et al., 2010). In addition, lower healthcare engagement was found to be an obstacle to routine mammography among transgender individuals and sexual minority (i.e., nonheterosexual) women; this is likely attributed to discrimination from providers (Bazzi et al., 2015). Patients were not asked about their SOGI in the clinical setting, leading to lack of cancer risk identification and resulting in poor rates of cervical cancer screening (Johnson, Mueller, et al., 2016).
Organization and/or practice setting and local community environment: LGBTQ populations perceived a lack of acceptance in the current healthcare delivery system. Most lesbian women were not asked about their SOGI when presenting for mammography screening for breast cancer (Johnson, Mueller, et al., 2016). The clinical delivery structure and system design contribute to barriers for cancer screening. In many studies examined in this review, participants reported fear of provider discrimination, unwelcoming environments, and discrimination when presenting to healthcare providers (Agénor et al., 2015; Austin et al., 2013; Bazzi et al., 2015; Blackwell & Eden, 2011; Butame et al., 2017; Johnson, Mueller, et al., 2016; Peitzmeier et al., 2017; Polek & Hardie, 2010; Reed et al., 2010; Tabaac et al., 2018; Thompson et al., 2015; Tracy et al., 2010). Negative experiences with healthcare providers led to nondisclosure of SOGI, which prevented the provision of accurate screening education (Johnson, Mueller, et al., 2016; Tabaac et al., 2018; Tracy et al., 2010). Heteronormative assumptions by providers, such as presuming their own sexuality or gender to be the norm, was a predominate theme leading to fear of SOGI disclosure (Agénor et al., 2015). Unwelcoming environments in healthcare offices and communities also perpetuated nondisclosure, discrimination, and poor screening adherence (Johnson, Mueller, et al., 2016; Tabaac et al., 2018; Tracy et al., 2010). In addition, regardless of gender, lesbian, bisexual, and queer patients seen by a gynecologist, as opposed to a nurse practitioner or a physician assistant, reported more negative experiences (Agénor et al., 2015).
State health policy environment and national health policy environment: Because most published screening guidelines are based on cisgender individuals, or those whose gender identity aligns with their sex assigned at birth (Human Rights Campaign, n.d.), recommendations do not consider the needs of transgender patients, and a lack of healthcare provider training in transgender health was identified (Peitzmeier et al., 2017; Tabaac et al., 2018). As a result, transgender patients did not have adequate knowledge of screening guidelines, which contributed to poor adherence (Blackwell & Eden, 2011; Butame et al., 2017; Polek & Hardie, 2010; Thompson et al., 2015; Tracy et al., 2010). Transgender male patients often need providers to advocate for them to insurance companies for coverage of cervical cancer screening because of their male gender identity but their need for what is typically thought of as a female screening test (Peitzmeier et al., 2017).
Facilitators to Cancer Screening Activity
Individual patient: In the studies reviewed, facilitators to cancer screening included biological factors, certain attitudes and behaviors, and socioeconomic factors. Mixed results regarding age and cancer screening were found in the reviewed studies. Although older age was a facilitator for obtaining mammography screening among individuals in sexual minority groups and transgender individuals (Bazzi et al., 2015), age was not correlated with lesbian women’s perceptions of barriers to being screened for cervical cancer (Tracy et al., 2010). When ethnicity or race, social position, life experiences, or gender matched that of their provider, Black lesbian, bisexual, and queer women were more likely to be comfortable, feel safe, and trust their provider (Agénor et al., 2015).
Transgender individuals who exhibited or perceived themselves as having feminine gender expression were more likely to adhere to routine cervical cancer screening than those who perceived themselves as being more masculine (Johnson, Mueller, et al., 2016). Willingness to pay for anal Pap tests was higher for gay and bisexual men who perceived or were worried that they had an increased risk of being diagnosed with anal cancer (Reed et al., 2010).
Routine cancer screening for patients was higher in those who placed importance on and kept routine appointments (Bazzi et al., 2015; Johnson, Mueller, et al., 2016). Lesbian, bisexual, or queer women who had a prior history of sexual activity or abnormal Pap test results were more likely to present for routine cervical cancer screening (Johnson, Mueller, et al., 2016). These facilitators are likely attributable to the younger age that women present for their first gynecologic examination because this becomes routine, with annual Pap tests automatically scheduled much of the time and reminders provided (Johnson, Mueller, et al., 2016).
Having any type of insurance coverage, higher income, private insurance, and employment positively influenced screening practices (Bazzi et al., 2015; Johnson, Mueller, et al., 2016). More education and higher income were associated with improved adherence to anal Pap tests by gay and bisexual men as well as cervical cancer screening by lesbian and bisexual women (Johnson, Mueller, et al., 2016; Reed et al., 2010; Tracy et al., 2010).
Family and social supports: Having a family history of cancer, positive role models, and peer support were identified as facilitators to cervical cancer screening adherence among lesbian and bisexual women (Johnson, Mueller, et al., 2016).
Provider/team: When providers did not assume heterosexuality and used open-ended questions regarding sexual orientation, lesbian and bisexual women were more comfortable undergoing a Pap test for cervical cancer screening (Agénor et al., 2015). Knowledge of same-sex sexual health; shared race, ethnicity, or socioeconomic status; and congruence in sexual orientation created increased comfort with screening in Black lesbian, bisexual, and queer women (Agénor et al., 2015).
Among transgender men and lesbian, bisexual, and queer women, gentle physical touch and acknowledgment of the possibility of discomfort were associated with positive screening experiences and increased adherence to routine screening (Agénor et al., 2015; Peitzmeier et al., 2017). Active listening to transgender patients and modification of the examination for comfort improved satisfaction with screening and decreased gender dysphoria (Peitzmeier et al., 2017).
When providers understood risk factors for anal cancer in men who have sex with men and were able to teach patients about self-examination, increased patient adherence to screening guidelines occurred (Thompson et al., 2015). Providing the option for self-collected versus physician-collected anal Pap test samples for rectal HPV improved the willingness of bisexual and gay men to undergo this screening (Thompson et al., 2015). Having received contraception or sexually transmitted infection services from a healthcare provider, a positive experience with the Pap test, a provider recommendation for cervical cancer screening, satisfaction with care, and “outness” to one’s provider were identified as facilitators to screening (Johnson, Mueller, et al., 2016). Lesbian and bisexual women and transgender men were found to be four times more likely to be screened routinely when such screening was recommended by their provider compared to those who did not receive a screening recommendation (Johnson, Mueller, et al., 2016). Those who reported routine cancer screening noted having good experiences with screening via Pap test (Johnson, Mueller, et al., 2016).
Organization and/or practice setting and local community environment: Lesbian and bisexual women reported that feeling welcomed by their provider and having their partner welcomed were key facilitators to adherence to cervical cancer screening (Johnson, Mueller, et al., 2016). In addition, when lesbian, bisexual, and queer women were seen by a physician assistant or nurse practitioner as opposed to a physician at a LGBTQ health clinic, they felt that more time and attention were given to them, resulting in greater satisfaction with the experience and increased likelihood to present for screening (Agénor et al., 2015). Men who have sex with men who were taught self- or partner anal examination reported that they had higher intentions of performing self-screening (Butame et al., 2017).
State health policy environment and national health policy environment: In states with legal protections related to sexual orientation, there was an 8% higher likelihood of having had colorectal cancer screening among LGBTQ populations, although this finding was not statistically significant (Austin et al., 2013).
Discussion
This review of 12 articles sought to identify the barriers and facilitators to cancer screening in LGBTQ populations using Whittemore and Knafl’s (2005) framework. Queer theory within the context of the MICCC framework was used to identify and categorize the themes that occurred frequently. Multiple barriers and facilitators to cancer screening among LGBTQ populations were identified and crossed many levels of influence; these included ways that heteronormativity influences patients’ adherence to cancer screening and providers’ treatment of LGBTQ populations. Determinants of health-seeking behavior included patients’ lack of knowledge regarding screening; federal, national, and individual discrimination; and absent cancer screening guidelines.
Patients’ lack of knowledge about screening guidelines was the most frequently cited barrier to cancer screening identified in this review. In a 2015 survey of 352 obstetricians and gynecologists across the United States by Unger (2015), 60% (n = 82) reported a lack of knowledge concerning guidelines for breast cancer screening in transgender populations, and only 29% (n = 41) reported being comfortable caring for transgender individuals (Pivo et al., 2017). This dearth of knowledge and feelings of discomfort likely stem from lack of provider training (Gonzales & Zinone, 2018; Pivo et al., 2017), bias against these populations (Foglia & Fredriksen-Goldsen, 2014; Fredriksen-Goldsen, Kim, Barkan, Muraco, & Hoy-Ellis, 2013; Khan, Plummer, Hussain, & Minichiello, 2008), and discrimination (Bradford, Reisner, Honnold, & Xavier, 2013: Braun et al., 2017; Burkhalter et al., 2016; Cochran et al., 2001). Although some states have laws banning discrimination based on SOGI, many do not and actively condone SOGI discrimination (Cahill, 2018). When LGBTQ patients perceived that providers created a welcoming environment, asked about SOGI status in a nonjudgmental way, were communicative, and encouraged teamwork between patients and providers, they were more likely to adhere to routine cancer screening (Agénor et al., 2015; Johnson, Mueller, et al., 2016; Peitzmeier et al., 2017).
The current political climate, lack of cancer screening guidelines specific to LGBTQ populations, and dearth of research and funding for LGBTQ-specific cancer data have led many in LGBTQ populations to fear discrimination from healthcare providers (Foglia & Fredriksen-Goldsen, 2014). This fear was the second most frequently identified barrier. Reasons that individuals may fear discrimination from providers may include provider age or historical context of the lived experiences of the patient and/or the provider (Foglia & Fredriksen-Goldsen, 2014), provider homophobia (Cochran et al., 2001; Tracy et al., 2010), patient transgender status (Bazzi et al., 2015; Boehmer & Elk, 2015; Bonvicini, 2017; Bristowe et al., 2018; Cahill, 2018; Fredriksen-Goldsen et al., 2013; Gibson, Radix, Maingi, & Patel, 2017; Peitzmeier et al., 2017; Tabaac et al., 2018), prior experience with provider or healthcare discrimination of patient (Cochran et al., 2001; Tracy et al., 2010), and unwelcoming healthcare environment (Cahill, 2018). Education regarding the provision of a safe environment should be required for providers, and the importance of obtaining a culturally competent health history should be emphasized because outness by LGBTQ populations is reported as being low (Bristowe et al., 2018; Johnson, Nemeth, et al., 2016). Additional research using larger-scale studies to assess cancer screening barriers and facilitators among LGBTQ populations, as well as to understand providers’ lack of comfort with and knowledge of such screening, should be prioritized. Cancer screening data among LGBTQ populations would lead to identification of areas to implement public interventions.
A major finding of this review was the small number of LGBTQ populations included in the studies. Of the participants across the reviewed studies, 1.5% self-reported as a sexual minority, and 0.04% identified as transgender or queer. The disproportionate ratios of heterosexual participants to LGBTQ populations in LGBTQ-specific studies highlight the need for larger studies with greater numbers of participants to determine population-based statistics.
Cancer screening data specifically focused on SOGI are not well represented in the literature. In a 2002 review of articles addressing health care among LGBTQ populations, it was found that only 0.1% of all articles in PubMed specifically addressed LGBTQ health–related subjects; in 2011, this number slightly increased to 0.3% (Institute of Medicine, 2011). In addition, of the LGBTQ healthcare research funded by the National Institutes of Health, 75% focuses on HIV, whereas only 1.8% of funding for this population is devoted to cancer (Gibson et al., 2017). Such findings suggest a need for federal resource allocation and funding for larger-scale studies to address these gaps (Agénor et al., 2015; Austin et al., 2013; Bazzi et al., 2015; Blackwell & Eden, 2011; Burkhalter et al., 2016; Dibble, Roberts, Robertson, & Paul, 2002; Gibson et al., 2017; Johnson, Mueller, et al., 2016; Peitzmeier et al., 2017; Quinn et al., 2015; Reed et al., 2010; Tabaac et al., 2018; Thompson et al., 2015).
Limitations
This review had several limitations. For example, findings lack generalizability to all LGBTQ groups or other diverse populations. In addition, the selection of search terms may have led to some applicable studies not being captured, although this was minimized by the use of the hand-search method and consultation of a medical reference librarian. Study samples also are comprised of individuals who are motivated to participate in research; therefore, individuals with increased discomfort or psychological distress related to disclosing their LGBTQ status might not be included. Also, themes that were identified through the review were extrapolated by one reviewer and may be subject to bias.
Implications for Nursing
The Institute of Medicine (2011), now known as the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine, has noted a lack of data to address the health needs of LGBTQ populations, and the Healthy People 2020 initiative, of the Office of Disease Prevention and Health Promotion (2019), has identified the importance of improving the health of LGBTQ populations. In addition, the National Institute on Minority Health and Health Disparities has classified LGBTQ populations as an area of disparity research (U.S. Department of Health and Human Services, 2016), and the American Nurses Association (ANA Ethics Advisory Board, 2019) recommends advocacy and protection for LGBTQ populations and the provision of culturally competent and inclusive care to all LGBTQ populations without discrimination.
With adequate training and knowledge, nurses have the potential to be pioneers in spearheading a variety of opportunities to reduce healthcare disparities among LGBTQ populations and ensure that LGBTQ individuals feel safe divulging their SOGI status in the healthcare setting. The Nurses’ Health Education About LGBTQ Elders cultural competency curriculum was developed as an institutional training program for nurses and other healthcare providers; providers who participated in the program demonstrated an increase in knowledge and cultural competency regarding the provision of care for LGBTQ populations (Hardacker, Rubinstein, Hotton, & Houlberg, 2014).
In addition, nurse-led research in this area aligns with the National Institute of Nursing Research’s strategic plan in which supporting wellness and prevention of illness is a priority for reducing disparities in sex and gender differences (Grady, 2017). Therefore, nurses are in a unique position to advocate for and attend to LGBTQ populations with the intention of improving communication, evaluating individual and environmental challenges, and offering education for all providers caring for this unique population. 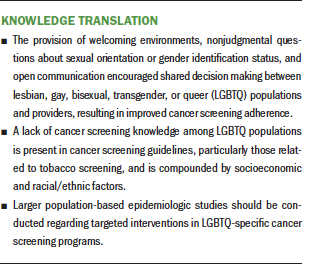
Conclusion
Based on the findings of this review, education and the provision of culturally sensitive health care for LGBTQ populations when presenting for cancer screening is imperative for improvement of the identified disparities. Although some research has been conducted, larger-scale population-based studies to understand the demographic and healthcare characteristics of cancer screening participation among LGBTQ populations need to be shepherded. This is essential to identify and address gaps in the literature, guide public health initiatives and interventions, and improve adherence to cancer screening (Johnson, Mueller, et al., 2016). Specifically, lack of knowledge by LGBTQ patients and providers about cancer screening guidelines; LGBTQ patients’ fear of discrimination; and screening discrepancies among patients of various socioeconomic, racial, and ethnic statuses should be the foci of future research.
About the Author(s)
Kelly S. Haviland, PhD, is a clinical nurse practitioner in the supportive care medicine inpatient service at Memorial Sloan Kettering Cancer Center in New York, NY; and Shannon Swette, PhD, is a professor, Teresa Kelechi, PhD, is the dean of research, and Martina Mueller, PhD, is a biostatistician, all in the College of Nursing at the Medical University of South Carolina in Charleston. No financial relationships to disclose. All authors contributed to the conceptualization and design and manuscript preparation. Haviland completed the data collection. Haviland, Swette, and Kelechi provided the analysis. Haviland can be reached at havilank@mskcc.org, with copy to ONFEditor@ons.org. (Submitted February 2019. Accepted July 3, 2019.)
References
Agénor, M., Bailey, Z., Krieger, N., Austin, S.B., & Gottlieb, B.R. (2015). Exploring the cervical cancer screening experiences of black lesbian, bisexual, and queer women: The role of patient-provider communication. Women and Health, 55, 717–736. https://doi.org/10.1080/03630242.2015.1039182
ANA Ethics Advisory Board. (2019). ANA position statement: Nursing advocacy for LGBTQ+ populations. OJIN, 24. https://doi.org/10.3912/OJIN.Vol24No01PoSCol02
Austin, S.B., Pazaris, M.J., Nichols, L.P., Bowen, D., Wei, E.K., & Spiegelman, D. (2013). An examination of sexual orientation group patterns in mammographic and colorectal screening in a cohort of U.S. women. Cancer Causes and Control, 24, 539–547. https://doi.org/10.1007/s10552-012-9991-0
Bazzi, A.R., Whorms, D.S., King, D.S., & Potter, J. (2015). Adherence to mammography screening guidelines among transgender persons and sexual minority women. American Journal of Public Health, 105, 2356–2358. https://doi.org/10.2105/AJPH.2015.302851
Berlant, L. (2006). Cruel optimism. Differences, 17(3), 20–36. https://doi.org/10.1215/10407391-2006-009
Blackwell, C.W., & Eden, C. (2011). Human papillomavirus and anorectal carcinoma knowledge in men who have sex with men. Journal of the Association of Nurses in AIDS Care, 22, 444–453. https://doi.org/10.1016/j.jana.2011.08.004
Boehmer, U., & Elk, R. (Eds.). (2015). Cancer and the LGBT community: Unique perspectives from risk to survivorship. Cham, Switzerland: Springer International Publishing.
Bonvicini, K.A. (2017). LGBT healthcare disparities: What progress have we made? Patient Education and Counseling, 100, 2357–2361. https://doi.org/10.1016/j.pec.2017.06.003
Bradford, J., Reisner, S.L., Honnold, J.A., & Xavier, J. (2013). Experiences of transgender-related discrimination and implications for health: Results from the Virginia Transgender Health Initiative Study. American Journal of Public Health, 103, 1820–1829. https://doi.org/10.2105/AJPH.2012.300796
Braun, H., Nash, R., Tangpricha, V., Brockman, J., Ward, K., & Goodman, M. (2017). Cancer in transgender people: Evidence and methodological considerations. Epidemiologic Reviews, 39, 93–107. https://doi.org/10.1093/epirev/mxw003
Bristowe, K., Hodson, M., Wee, B., Almack, K., Johnson, K., Daveson, B.A., . . . Harding, R. (2018). Recommendations to reduce inequalities for LGBT people facing advanced illness: ACCESSCare national qualitative interview study. Palliative Medicine, 32, 23–35. https://doi.org/10.1177/0269216317705102
Brown, A. (2017, June 13). 5 key findings about LGBT Americans. Retrieved from http://www.pewresearch.org/fact-tank/2017/06/13/5-key-findings-about-lg…
Burkhalter, J.E., Margolies, L., Sigurdsson, H.O., Walland, J., Radix, A., Rice, D., . . . Maingi, S. (2016). The national LGBT Cancer Action Plan: A white paper of the 2014 National Summit on Cancer in the LGBT communities. LGBT Health, 3, 19–31. https://doi.org/10.1089/lgbt.2015.0118
Butame, S.A., Lawler, S., Hicks, J.T., Wilkerson, J.M., Hwang, L.-Y., Baraniuk, S., . . . Nyitray, A.G. (2017). A qualitative investigation among men who have sex with men on the acceptability of performing a self- or partner anal exam to screen for anal cancer. Cancer Causes and Control, 28, 1157–1166. https://doi.org/10.1007/s10552-017-0935-6
Butler, J. (2008). Gender trouble: Feminism and the subversion of identity. New York, NY: Routledge.
Cahill, S.R. (2018). Legal and policy issues for LGBT patients with cancer or at elevated risk of cancer. Seminars in Oncology Nursing, 34, 90–98. https://doi.org/10.1016/j.soncn.2017.12.006
Cochran, S.D., Mays, V.M., Bowen, D., Gage, S., Bybee, D., Roberts, S.J., . . . White, J. (2001). Cancer-related risk indicators and preventive screening behaviors among lesbians and bisexual women. American Journal of Public Health, 91, 591–597. https://doi.org/10.2105/ajph.91.4.591
Daniel, H., & Butkus, R. (2015). Lesbian, gay, bisexual, and transgender health disparities: Executive summary of a policy position paper from the American College of Physicians. Annals of Internal Medicine, 163, 135–137. https://doi.org/10.7326/M14-2482
Dibble, S.L., Roberts, S.A., Robertson, P.A., & Paul, S.M. (2002). Risk factors for ovarian cancer: Lesbian and heterosexual women [Online exclusive]. Oncology Nursing Forum, 29, E1–E7. https://doi.org/10.1188/02.ONF.E1-E7
Edelman, L. (2004). No future: Queer theory and the death drive. Durham, NC: Duke University Press.
Foglia, M.B., & Fredriksen-Goldsen, K.I. (2014). Health disparities among LGBT older adults and the role of nonconscious bias. Hastings Center Report, 44(Suppl. 4), S40–S44. https://doi.org/10.1002/hast.369
Foucault, M. (1995). Madness, the absence of work (P. Stastny & D. Şengel, Trans.). Chicago, IL: University of Chicago Press.
Fredriksen-Goldsen, K.I., Kim, H.-J., Barkan, S.E., Muraco, A., & Hoy-Ellis, C.P. (2013). Health disparities among lesbian, gay, and bisexual older adults: Results from a population-based study. American Journal of Public Health, 103, 1802–1809. https://doi.org/10.2105/AJPH.2012.301110
Gibson, A.W., Radix, A.E., Maingi, S., & Patel, S. (2017). Cancer care in lesbian, gay, bisexual, transgender and queer populations. Future Oncology, 13, 1333–1344. https://doi.org/10.2217/fon-2017-0482
Giffney, N. (2004). Denormatizing queer theory: More than (simply) lesbian and gay studies. Feminist Theory, 5, 73–78. https://doi.org/10.1177%2F1464700104040814
Gonzales, G., & Zinone, R. (2018). Cancer diagnoses among lesbian, gay, and bisexual adults: Results from the 2013–2016 National Health Interview Survey. Cancer Causes and Control, 29, 845–854. https://doi.org/10.1007/s10552-018-1060-x
Grady, P.A. (2017). Advancing science, improving lives: NINR’s new strategic plan and the future of nursing science. Journal of Nursing Scholarship, 49, 247–248. https://doi.org/10.1111/jnu.12286
Gruskin, E.P., Hart, S., Gordon, N., & Ackerson, L. (2001). Patterns of cigarette smoking and alcohol use among lesbians and bisexual women enrolled in a large health maintenance organization. American Journal of Public Health, 91, 976–979. https://doi.org/10.2105/ajph.91.6.976
Halberstam, J. (2005). In a queer time and place: Transgender bodies, subcultural lives. New York, NY: New York University Press.
Hardacker, C.T., Rubinstein, B., Hotton, A., & Houlberg, M. (2014). Adding silver to the rainbow: The development of the Nurses’ Health Education About LGBT Elders (HEALE) cultural competency curriculum. Journal of Nursing Management, 22, 257–266. https://doi.org/10.1111/jonm.12125
Harris, J., & White, V. (2018). A dictionary of social work and social care. Oxford, UK: Oxford University Press.
Human Rights Campaign. (n.d.). Glossary of terms. Retrieved from https://www.hrc.org/resources/glossary-of-terms
Institute of Medicine. (2011). The health of lesbian, gay, bisexual, and transgender people: Building a foundation for better understanding. Washington, DC: National Academies Press.
Jagose, A. (1997). Queer theory: An introduction. New York, NY: New York University Press.
Johnson, M.J., Mueller, M., Eliason, M.J., Stuart, G., & Nemeth, L.S. (2016). Quantitative and mixed analyses to identify factors that affect cervical cancer screening uptake among lesbian and bisexual women and transgender men. Journal of Clinical Nursing, 25, 3628–3642. https://doi.org/10.1111/jocn.13414
Johnson, M.J., Nemeth, L.S., Mueller, M., Eliason, M.J., & Stuart, G.W. (2016). Qualitative study of cervical cancer screening among lesbian and bisexual women and transgender men. Cancer Nursing, 39, 455–463. https://doi.org/10.1097/NCC.0000000000000338
Khan, A., Plummer, D., Hussain, R., & Minichiello, V. (2008). Does physician bias affect the quality of care they deliver? Evidence in the care of sexually transmitted infections. Sexually Transmitted Infections, 84, 150–151. https://doi.org/10.1136/sti.2007.028050
McCabe, S.E., West, B.T., Hughes, T.L., & Boyd, C.J. (2013). Sexual orientation and substance abuse treatment utilization in the United States: Results from a national survey. Journal of Substance Abuse Treatment, 44, 4–12. https://doi.org/10.1016/j.jsat.2012.01.007
McComiskey, C., Simone, S., Schofield, D., McQuillan, K., Andersen, B., Johannes, S., & Weichold, A. (2018). Professional advancement for advanced practice clinicians. Journal for Nurse Practitioners, 14, 12–17.e5. https://doi.org/10.1016/j.nurpra.2017.09.018
Newport, F. (2018, May 22). In U.S., estimate of LGBT population rises to 4.5%. Retrieved from https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
Office of Disease Prevention and Health Promotion. (2019). Lesbian, gay, bisexual, and transgender health. Retrieved from https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-…
Parekh, R. (2016). What is gender dysphoria? Retrieved from https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-g…
Peitzmeier, S.M., Agénor, M., Bernstein, I.M., McDowell, M., Alizaga, N.M., Reisner, S.L., . . . Potter, J. (2017). “It can promote an existential crisis”: Factors influencing Pap test acceptability and utilization among transmasculine individuals. Qualitative Health Research, 27, 2138–2149. https://doi.org/10.1177/1049732317725513
Pivo, S., Montes, J., Schwartz, S., Chun, J., Kiely, D., Hazen, A., & Schnabel, F. (2017). Breast cancer risk assessment and screening in transgender patients. Clinical Breast Cancer, 17, e225–e227. https://doi.org/10.1016/j.clbc.2016.08.003
Polek, C., & Hardie, T. (2010). Lesbian women and knowledge about human papillomavirus [Online exclusive]. Oncology Nursing Forum, 37, E191–E197. https://doi.org/10.1188/10.ONF.E191-E197
Quinn, G.P., Sanchez, J.A., Sutton, S.K., Vadaparampil, S.T., Nguyen, G.T., Green, B.L., . . . Schabath, M.B. (2015). Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA: A Cancer Journal for Clinicians, 65, 384–400. https://doi.org/10.3322/caac.21288
Reed, A.C., Reiter, P.L., Smith, J.S., Palefsky, J.M., & Brewer, N.T. (2010). Gay and bisexual men’s willingness to receive anal Papanicolaou testing. American Journal of Public Health, 100, 1123–1129. https://doi.org/10.2105/AJPH.2009.176446
Sedgwick, E.K. (2005). Epistemology of the closet. Berkeley, CA: University of California Press.
Stokols, D. (1996). Translating social ecological theory into guidelines for community health promotion. American Journal of Health Promotion, 10, 282–298. https://doi.org/10.4278/0890-1171-10.4.282
Tabaac, A.R., Sutter, M.E., Wall, C.S.J., & Baker, K.E. (2018). Gender identity disparities in cancer screening behaviors. American Journal of Preventive Medicine, 54, 385–393. https://doi.org/10.1016/j.amepre.2017.11.009
Tang, H., Greenwood, G.L., Cowling, D.L., Lloyd, J.C., Roeseler A.G., & Bal, D.G. (2004). Cigarette smoking among lesbians, gays, and bisexuals: How serious a problem? (United States). Cancer Causes and Control, 15, 797–803. https://doi.org/10.1023/B:CACO.0000043430.32410.69
Taplin, S.H., Anhang Price, R., Edwards, H.M., Foster, M.K., Breslau, E.S., Chollette, V., . . . Zapka, J. (2012). Introduction: Understanding and influencing multilevel factors across the cancer care continuum. Journal of the National Cancer Institute. Monographs, 2012(44), 2–10. https://doi.org/10.1093/jncimonographs/lgs008
Thompson, J.A., Reiter, P.L., McRee, A.-L., Moss, J.L., & Brewer, N.T. (2015). Gay and bisexual men’s willingness to use a self-collected anal cancer screening test. Journal of Lower Genital Tract Disease, 19, 354–361. https://doi.org/10.1097/LGT.0000000000000118
Tracy, J.K., Lydecker, A.D., & Ireland, L. (2010). Barriers to cervical cancer screening among lesbians. Journal of Women’s Health, 19, 229–237. https://doi.org/10.1089/jwh.2009.1393
Tuhkanen, M. (Ed.). (2014). Leo Bersani: Queer theory and beyond. Albany, NY: State University of New York Press.
Unger, C.A. (2015). Care of the transgender patient: A survey of gynecologists’ current knowledge and practice. Journal of Women’s Health, 24, 114–118. https://doi.org/10.1089/jwh.2014.4918
U.S. Department of Health and Human Services. (2016). Advancing LGBT health and well-being. Retrieved from https://www.hhs.gov/sites/default/files/2016-report-with-cover.pdf
Whittemore, R., & Knafl, K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52, 546–553. https://doi.org/10.1111/j.1365-2648.2005.03621.x
Worthen, M.G.F. (2016). Sexual deviance and society: A sociological examination. Abingdon, UK: Routledge.



