Hyperglycemia and Cancer: A State-of-the-Science Review
Problem Identification: Hyperglycemia can increase the risk for adverse events and outcomes in patients undergoing treatment for cancer. The purposes of this state-of-the-science review were to explore the complexity of hyperglycemia in patients with cancer and to analyze physiologic mechanisms and outcomes in individuals with or at risk for cancer.
Literature Search: PubMed® and the Cochrane Library databases were searched, and 95 articles were included. Findings were evaluated for their methods and analyses. Studies assessed as methodologically flawed were not included.
Data Evaluation: The synthesis of the articles provided the evidence for describing normal and glycemic pathways. Hyperglycemia in patients with cancer was explored through chronic inflammatory mechanisms that lead to increased risks for adverse events and outcomes.
Synthesis: This article discusses normal glucose regulation and hyperglycemic pathways, hyperglycemia in patients with cancer, hyperglycemia and cancer-related inflammation, and outcomes (e.g., infections, mortality, symptoms).
Implications for Research: Understanding the contributors to and consequences of hyperglycemia can guide the development of screening tools to predict which individuals are at the greatest risk for hyperglycemic episodes prior to starting cancer therapies. Research can lead to glycemic guidelines specific to patients with cancer for better outcomes.
Jump to a section
In 2010, a joint statement between the American Diabetes Association (ADA) and American Cancer Society detailed the epidemiologic evidence for the increased risk for certain cancers among individuals with type 2 diabetes (T2D) (Giovannucci et al., 2010). Specifically, preexisting T2D was found to be associated with increased risk for cancers of the liver, pancreas, and endometrium, as well as, to a lesser degree, cancers of the colon, rectum, breast, bladder, and lung (Giovannucci et al., 2010). In alignment, the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System estimated that the overall prevalence of preexisting T2D is 16.7% among cancer survivors (Underwood et al., 2012). In comparison, the prevalence of T2D in the general U.S. population is 9.4% (ADA, 2018b). This evidence highlights the increased risk for developing cancer among those with preexisting T2D and demonstrates the high prevalence of T2D among survivors of cancer.
The hallmark characteristic of T2D is hyperglycemia, which is defined as a random blood glucose (BG) of 126 mg/dl or greater or a fasting BG of greater than 100 mg/dl (ADA, 2018a). Hyperglycemia can also occur at a pre-T2D level, which is a higher-than-normal BG level but has not reached the threshold for a diagnosis of T2D (ADA, 2018a; Anil, Akkurt, Ayturk, Kut, & Gursoy, 2013). Among individuals undergoing treatment for cancer, hyperglycemic episodes can occur with or without having T2D or pre-T2D and can increase the risk for adverse events. For example, one study examined differences between hyperglycemic episodes in individuals with or without T2D undergoing treatment for cancer and found that decreased overall survival was associated with episodes of hyperglycemia rather than the diabetes diagnosis (Villarreal-Garza et al., 2012). This finding suggests that poor glycemic control may be a greater risk for adverse events and outcomes than having a diagnosis of T2D with good glycemic control.
Several adverse events and outcomes associated with hyperglycemia during cancer therapies have been noted, including increased risks of infections (Derr, Hsiao, & Saudek, 2008; Fuji et al., 2007; Hammer et al., 2016; Storey & Von Ah, 2016), toxicity (Brunello, Kapoor, & Extermann, 2011), longer hospital length of stay (Storey & Von Ah, 2015), chemoresistance (Biernacka et al., 2013; Zeng et al., 2010), cancer recurrence (Giacco & Brownlee, 2010; Wright et al., 2013), cancer progression and metastasis (Duan et al., 2014; Li et al., 2012), and decreased overall and disease-free survival (Barua et al., 2018; Hammer et al., 2009; Villarreal-Garza et al., 2012; Zhou et al., 2010).
Taken together, hyperglycemia at a level that defines the diagnosis of T2D increases the risk for a cancer diagnosis, and hyperglycemic episodes with or without a diagnosis of T2D (or pre-T2D) increases the risk for adverse events and outcomes in individuals undergoing treatment for cancer. Understanding the complex physiologic mechanisms associated with hyperglycemia in the cancer environment is imperative to facilitate early identification of high-risk individuals. Preemptive interventions can be implemented throughout the cancer trajectory to mitigate the deleterious impact of hyperglycemia for improved outcomes and overall quality of life in individuals undergoing treatment for cancer. The purposes of this review were to explore the complexity of hyperglycemia in patients with cancer and to analyze physiologic mechanisms and outcomes in individuals with or at risk for cancer. This foundation can inform future research and practice for improved patient care and outcomes.
Literature Search
This state-of-the-science review followed the PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-Analysis) statement guidelines to identify, select, and critically appraise the relevant literature (Liberati et al., 2009). PubMed® and the Cochrane Library databases were searched using combinations of search terms, including hyperglycemia, cancer, type 2 diabetes, metabolic syndrome, body mass index, chemotherapy, glucocorticoids, symptoms, organ dysfunction, death, and mortality. An initial 12,043 peer-reviewed journal articles were identified. After removing duplicates (n = 2,293), articles that did not include specific mechanisms (n = 8,557), or studies that were methodologically flawed (n = 1,098), a final 95 articles were included in this review.
Information from the included articles was synthesized to describe the evidence for normal and hyperglycemic pathways. Hyperglycemia in patients with cancer was explored through chronic inflammatory mechanisms that lead to increased risks for adverse events and outcomes.
Synthesis
The purposes of this review were to explore the complexity of hyperglycemia in individuals with cancer and analyze physiologic mechanisms and outcomes in individuals with or at risk for cancer. The following scientific review of the literature addresses these goals through a systematic approach. First, normal glucose regulation and hyperglycemic pathways are described. Hyperglycemia in patients with cancer is then introduced, first with a brief overview of the contributors to hyperglycemia. Hyperglycemia and cancer-related inflammation are then explored. Finally, outcomes including infections, mortality, and symptoms are discussed. Table 1 provides a list of select elements involved in biophysiologic pathways. 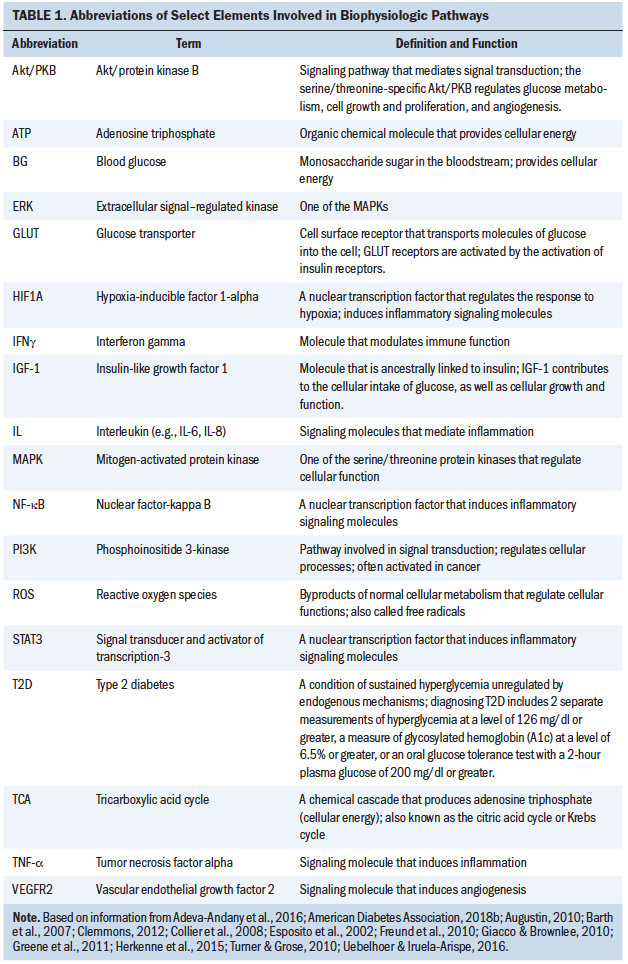
Glycemic Pathways
Glucose regulation: Glucose is a monosaccharide sugar that is essential for cellular functioning. Distributed through the vascular circuitry, the therapeutic range of BG is 4–6 mmol (72.07–108.11 mg/dl) (Roder, Wu, Liu, & Han, 2016). Maintaining this relatively tight range requires a multilevel orchestration of biomolecular activity. Glucose is obtained through three primary sources: intestinal absorption of carbohydrates through digestion, glycogenolysis (conversion of glycogen stores in the liver to glucose), and gluconeogenesis (formation of new glucose in the liver from protein storages in the muscles) (Adeva-Andany, Pérez-Felpete, Fernández-Fernández, Donapetry-García, & Pazos-García, 2016; Roder et al., 2016). The regulation of glucose occurs systemically through the signaling of regulatory mechanisms that initiate the pancreatic release of the hormones glucagon from the alpha cells and insulin from the beta cells. In addition to glucagon, catecholamine’s (epinephrine, norepinephrine, cortisol, and growth hormones) elevate BG through stimulation of glycogenolysis, gluconeogenesis, and inhibition of insulin uptake by the cells (Barth et al., 2007). Insulin, a key regulator of glucose’s entry into cells, activates glucose transporter (GLUT) receptors and promotes synthesis of glycogen (Augustin, 2010; Kristensen, Fredholm, & Cirera, 2015; Uebelhoer & Iruela-Arispe, 2016). Insulin also stimulates glycogen formation. Growth hormone and insulin-like growth factor 1 (IGF-1) are two key regulators of cellular growth and functioning (Werner, Sarfstein, LeRoith, & Bruchim, 2016). IGF-1 is also regulated by insulin and growth hormone, triggered by hepatic regulation from protein and energy (Clemmons, 2012). The IGF-1 receptor is similar to insulin receptors and, like insulin, contributes to the uptake of glucose into cells (Clemmons, 2012; Werner et al., 2016). Glucokinase, a regulatory protein found in multiple tissues (Massa, Gagliardino, & Francini, 2011), and neuroendocrine activity from the hypothalamic–pituitary–adrenal (HPA) axis (Kalsbeek et al., 2010) also contribute to the regulation of BG. The HPA axis is a feedback loop that triggers the release of epinephrine, norepinephrine, and cortisol (Dombrowski & Karounos, 2013), which promote gluconeogenesis.
Upon entry into the cell, glucose is metabolized in the mitochondria through the tricarboxylic acid cycle, also known as the Krebs cycle, for the production of adenosine triphosphate (ATP), which is cellular energy (Brownlee, 2005; Giacco & Brownlee, 2010; Yu, Jhun, & Yoon, 2011). Normal glucose metabolism yields 32–36 molecules of ATP through an aerobic respiratory pathway that includes oxidative phosphorylation (Asgari, Zabihinpour, Salehzadeh-Yazdi, Schreiber, & Masoudi-Nejad, 2015). Specifically, in normal oxygenated cells, glucose is converted into pyruvate (Uebelhoer & Iruela-Arispe, 2016; Yeluri, Madhok, Prasad, Quirke, & Jayne, 2009), which triggers an electrochemical gradient across the electron transport chain, resulting in positively charged ions that initiate the phosphorylation of adenosine diphosphate to create ATP via ATP synthase (Adeva-Andany et al., 2016; Giacco & Brownlee, 2010; Yu et al., 2011). The production of energy is coupled with consumption of oxygen, which is normally reduced to water, with 4%–5% being converted to reactive oxygen species (ROS), also called free radicals (Klaunig & Kamendulis, 2004). ROS are byproducts of normal cell metabolism used to regulate and maintain physiologic cell functions, including proliferation, migration, differentiation, senescence, apoptosis, signal transduction, and gene expression (Zhou, Shao, & Spitz, 2014). ROS, in turn, are regulated by antioxidants that contribute to cellular homeostasis (Zhou et al., 2014).
Hyperglycemia: Hyperglycemia occurs when the body’s regulatory mechanisms cannot maintain glucose below 6 mmol (108.11 mg/dl) (Roder et al., 2016). Hyperglycemia initiates the release of cortisol, catecholamines (epinephrine and norepinephrine), glucagon, and growth hormone, which leads to increased insulin resistance, hyperinsulinemia, lipolysis, gluconeogenesis, and glycogenolysis, further promoting hyperglycemia (Smiley & Umpierrez, 2010). The insulin resistance, characterized by needing higher-than-normal levels of insulin to facilitate the transport of circulating glucose into the cells (Park, Park, & Sweeney, 2015), initiates a deleterious cascade. In effect, the GLUT receptors become less efficient in responding to circulating insulin and IGF-1 triggers (Becker, Dossus, & Kaaks, 2009; Clemmons, 2012). In response, states of hyperinsulinemia and glyconeogenesis occur (Becker et al., 2009; Clemmons, 2012; Park et al., 2015). In addition, in the presence of hyperglycemia, excessive amounts of ROS are produced, creating oxidative stress (Yu et al., 2011). Hyperglycemia also stimulates excessive levels of calcium to enter the cell, which causes mitochondrial fragmentation, also leading to high levels of ROS and associated oxidative stress. The oxidative stress from these pathways interrupts normal cell metabolism, cell pathway signaling, and cell-to-cell homeostasis (Yu et al., 2011; Ziech et al., 2010). The downstream effect is a nuclear signaling cascade that evokes the activation of transcription factors, including signal transducer and activator of transcription 3 (STAT3), hypoxia-inducible factor 1-alpha (HIF1A), and nuclear factor-kappa B (NF-kB). These transcription factors induce an overexpression of inflammatory signaling molecules, including cytokines, chemokines, and prostaglandins (Esposito et al., 2002). Studies have found interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-a), and interferon gamma (IFNg) expressed at pathologically high levels (Esposito et al., 2002; Gale, Sicoutris, Reilly, Schwab, & Gracias, 2007; Germenis & Karanikas, 2007; Kumar & Gabrilovich, 2014). Other cytokine mediators of inflammation (IL-8, IL-10, and IL-18) may also be involved. The overexpression of these inflammatory signaling molecules inhibits immune function. Cellular activities, such as complement fixation, cell adherence, chemotaxis, phagocytosis, and apoptosis, are impaired, preventing detection and elimination of infectious microorganisms and allowing infections to manifest and thrive, a contributing factor to death among patients with cancer (Butler, Btaiche, & Alaniz, 2005; Collier, Dossett, May, & Diaz, 2008). Figure 1 is an overview of some of these pathways. 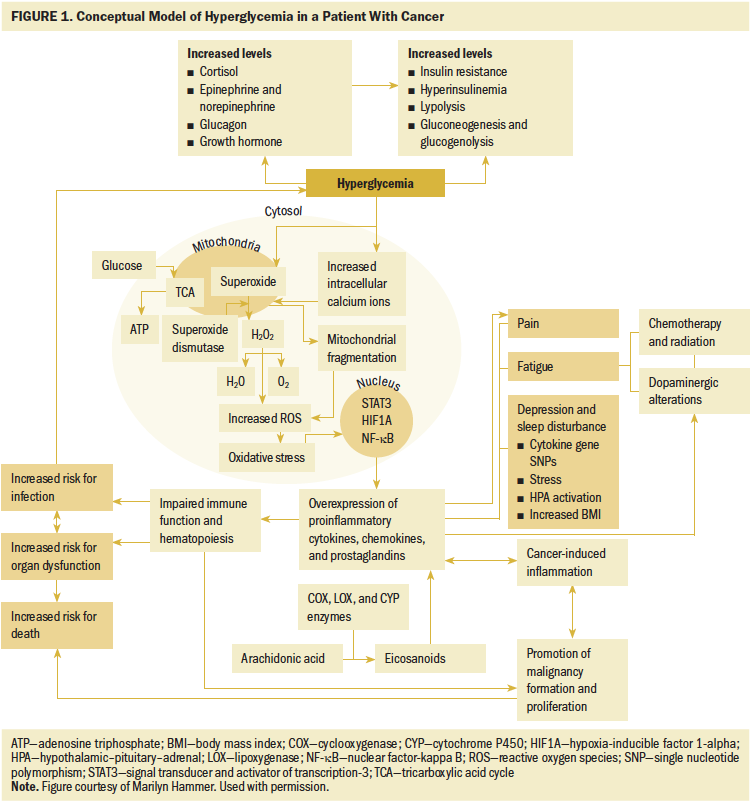
Confluence of Hyperglycemia and Cancer
The complex associations among hyperglycemia, increased risk for cancer, and increased risk for adverse outcomes in patients undergoing treatment for cancer share common chronic inflammatory mechanisms. Understanding the contributors to hyperglycemia and inflammation, as well as the pathways leading to or in the presence of cancer, can help to identify targets for improved outcomes and overall quality of life.
Contributors to hyperglycemia in patients with cancer: There are multiple contributors to hyperglycemia in patients undergoing treatment for cancer. Known and likely contributors include a preexisting high-calorie, high-fat, and high-sugar diet (overnutrition and/or poor-nutrient diet) (Hardman, 2014), low physical activity (Bird & Hawley, 2012, 2017), high consumption of alcohol, use of tobacco and other substances, and patient factors, such as being older aged (Freund, Orjalo, Desprez, & Campisi, 2010), having comorbid conditions, having a high body mass index (BMI) (Giovannucci et al., 2010), and, in some cases, having a related metabolic syndrome (Zafar, Khaliq, Ahmad, Manzoor, & Lone, 2018). BMI is reflective of behaviors and physiologic body function and has been implicated as a risk factor for cancer, T2D, and cardiovascular disease (de Mutsert, Sun, Willett, Hu, & van Dam, 2014; Zheng et al., 2014). Stress is also a potential contributor to hyperglycemia and inflammatory responses (Collier et al., 2008), leading to increased risks for adverse events in patients undergoing treatment for cancer. This pathway was clinically highlighted in studies that found that individuals with T2D-related distress had worse glycemic control and higher levels of glycosylated hemoglobin (A1c) (Aikens, 2012; Fisher et al., 2013; Fisher, Hessler, Polonsky, & Mullan, 2012; Snoek, Bremmer, & Hermanns, 2015). In addition, some medications, such as certain chemotherapy agents (Hershey et al., 2014) and glucocorticoids (Brady et al., 2014), induce hyperglycemia. Some treatments, such as hematopoietic cell transplantation (HCT) and related conditioning regimens, also increase the risk for post-treatment onset of T2D (Fuji, Löffler, Savani, Einsele, & Kapp, 2017). As shown in Figure 2, these factors contribute to chronic states of inflammation and hyperglycemic-induced inflammation. 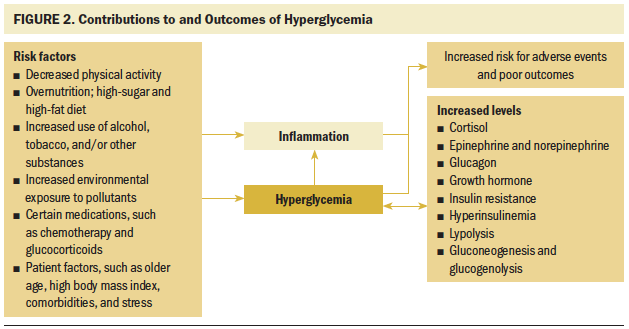
Hyperglycemic- and cancer-related inflammation in cancer formation and progression: Associations between glucose and cancer have been investigated for nearly a century (Holmes, 1940). By the 1960s, the specific influence of hyperglycemia on immune function (i.e., inflammatory response) in cancer formation and progression was described (Scott, 1968; Scott & Still, 1968; Stjernholm, 1967). This mechanism includes persistent oxidative stress, common in hyperglycemia and cancer, that leads to chronic inflammation, which compromises the immune system’s ability to detect and eliminate cancer-forming cells and, therefore, mediating the development and progression of cancer (Calle & Fernandez, 2012; Mantovani, Allavena, Sica, & Balkwill, 2008). Hyperglycemia also promotes an acidic milieu that provides a favorable microenvironment where malignant cells can thrive (Kellenberger et al., 2010). The metabolism of cancerous cells differs from normal healthy cells with preferential use of the lactate dehydrogenase pathway, using glycolysis for energy instead of oxidative phosphorylation, the pathway used in healthy cells (San-Millán & Brooks, 2016). Termed the Warburg Effect, cancer cells’ preference toward the lactate dehydrogenase pathway, even in well-oxygenated environments, is likely because of lactate activating HIF1A, which increases vascular endothelial growth factor 2 (VEGFR2), a promoter of angiogenesis (Herkenne et al., 2015). HIF1A also upregulates fibroblast growth factor, which promotes cancer cell progression (Turner & Grose, 2010). Another theory for the preferential use of lactate dehydrogenase by cancer cells is that apoptosis may be circumvented via the production of ROS (Manoochehri Khoshinani, Afshar, & Najafi, 2016; Yeluri et al., 2009) (see Table 2). 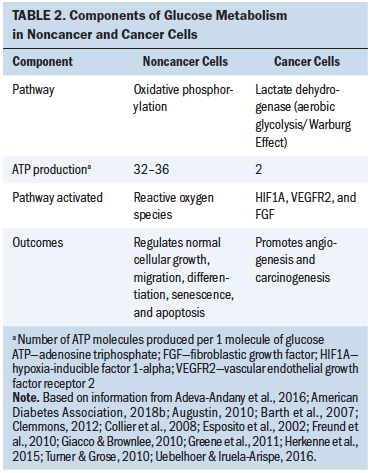
Other studies have uncovered the interactions among various ligands and receptors related to these pathways. GLUT receptors, for example, are overexpressed in cancers of the esophagus, stomach, colon, pancreas, lung, brain, ovaries, breast, and prostate (Yeluri et al., 2009). In addition, GLUT receptors are found on some cancerous tissues in which they are not normally expressed in the same cancer-naive tissue. GLUT3, for example, is normally expressed in brain tissue but is found in high abundance in cancerous tissues of the ovaries, lung, and stomach (Yeluri et al., 2009). In effect, more glucose is solicited to these tissues, with an associated inflammatory effect that alters cell signaling from oxidative stress and increases DNA damage and mutations (Giacco & Brownlee, 2010; Yeluri et al., 2009; Ziech et al., 2010).
Insulin, needed for GLUT receptor expression, glycogen synthesis, glycolysis, and fatty acid synthesis, also promotes carcinogenesis through several mechanisms. Insulin can aberrantly activate the serine/threonine-specific protein kinase called protein kinase B (PKB) and also known as Akt, a regulator of glucose metabolism, cell growth and proliferation, and angiogenesis (Testa & Tsichlis, 2005). Insulin can also stimulate mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/Akt, and extracellular signal–regulated kinase (ERK), all of which can promote malignant tissue through proliferation, invasiveness, angiogenesis, and inhibition of apoptosis (de Boer, Wörner, Verlaan, & van Leeuwen, 2017). IGF-1 is not only similar to insulin, but also ancestrally linked (Werner, Weinstein, & Bentov, 2008). In addition, IGF-1 regulates cellular growth, development, and metabolism and promotes metastases of malignant tissue through epithelial–mesenchymal transition (Li et al., 2017). Because of these associations, IGF-1 receptor is being studied as a possible target for cancer therapies (Li et al., 2017).
Hyperglycemia and adverse events in patients with cancer: Several studies have shown the increased risks of infections (Derr et al., 2008; Fuji et al., 2007; Hammer et al., 2016), organ dysfunction (Fuji et al., 2007), and mortality (Hammer et al., 2009; Zhou et al., 2010; Zhou, Zhang, Gu, & Xia, 2015) among patients with cancer who experience hyperglycemic episodes. Particularly in patients undergoing HCT, infection risk is increased 1.1–1.6 times with hyperglycemic episodes (Derr et al., 2008; Hammer et al., 2009, 2016). The risk for mortality in patients with hyperglycemia can be as high as 1.9–2.9 times the risk of those who do not become hyperglycemic (Fuji et al., 2007; Hammer et al., 2009). The impaired immune function related to an overexpression of inflammatory signaling molecules is a likely pathway toward the increased risk for these adverse events and outcomes (Esposito et al., 2002; Gale et al., 2007; Germenis & Karanikas, 2007; Kumar & Gabrilovich, 2014). This association was somewhat captured in a small study of collected blood samples from individuals without T2D who underwent autologous HCT. Inflammatory cytokines (e.g., IL-1b, IL-6, TNF-a) were elevated in individuals with infections compared to individuals without infections. Those with infections also had higher BG levels (Hammer et al., 2016). Although the cytokines (inflammatory markers) should have solicited an increase in white blood cells (WBCs) to eradicate the microorganisms, the patients with infections and higher cytokine expression had a lower WBC response than individuals without infections. Larger studies that measure these and other biomarkers are warranted to best understand these mechanisms.
Other mechanisms under investigation are related to symptoms. Symptoms from cancer and cancer therapies are highly prevalent and can be as devastating as or worse than the cancer diagnosis itself (Pud et al., 2008). Symptom severity is also inversely related to functional status and quality of life (Miaskowski et al., 2006; Pud et al., 2008) and may be predictive of survival (Teunissen, de Graeff, de Haes, & Voest, 2006). Some mechanisms suggest that hyperglycemia may be associated with greater symptom severity for certain symptoms.
Possible pathways exist for associations between hyperglycemia and greater levels of pain, fatigue, depression, and sleep disturbance—symptoms that often co-occur in individuals undergoing treatment for cancer (Miaskowski, 2016; Miaskowski et al., 2017). Patients with hyperglycemia and cancer may experience more severe or compounded symptoms because of the exacerbated inflammation. In addition, inflammation is associated with hyperglycemia and with factors that contribute to hyperglycemia. As described previously, BMI can be a significant contributor. Being overweight or obese creates a chronic systemic inflammatory state that can promote development and/or exacerbation of symptoms (Lasselin & Capuron, 2014; Rodríguez-Hernández, Simental-Mendía, Rodríguez-Ramírez, & Reyes-Romero, 2013). The influence of inflammation has also been found on a genetic level. For example, a study by Wright et al. (2017) found associations between inflammatory pathway genes and interindividual variability in the trajectories of fatigue in patients with solid tumor cancers receiving chemotherapy. The complexities of interindividual variability and how symptom experiences can be influenced by multiple factors underscore the need for understanding the mechanisms of modifiable factors, such as BG.
The experience of pain among individuals undergoing treatment for cancer can vary and be influenced by many factors. Understanding the mechanisms that contribute to pain pathways in this population is essential. One mechanism includes the production of eicosanoid metabolites from arachidonic acid by cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes (Wang et al., 2018). Subsequently, prostaglandins and leukotrienes are released, promoting an inflammatory response and associated pain (Greene, Huang, Serhan, & Panigrahy, 2011).
For some patients, pain may be related to neurotoxic chemotherapies that create peripheral neuropathy, a symptom also experienced by individuals with T2D. It is sometimes difficult to distinguish the cause of peripheral neuropathy in patients with T2D who receive neurotoxic chemotherapy. For some patients, the peripheral neuropathy is compounded by both. Because chemotherapy-induced peripheral neuropathy may necessitate early treatment termination or reduced dosing (Beijers, Mols, & Vreugdenhil, 2014), controlling this symptom when it is related to glucose is imperative.
Mechanistically, hyperglycemia in patients with T2D increases the rate of nerve cell death and impairs the cells’ repair mechanism (Tavakoli, Mojaddidi, Fadavi, & Malik, 2008). Chemotherapy agents, specifically those that bind to and disrupt microtubules (e.g., taxanes, vinca alkaloids, epothilones, oxaliplatin) cause nerve cell death and related neurotoxic effects (Donovan, 2009), increasing the risk for peripheral neuropathy (Pachman et al., 2016). Patients with cancer who are receiving a neurotoxic agent and experiencing hyperglycemia are potentially at higher risk for developing numbness and tingling and experiencing higher levels of severity of peripheral neuropathy (Hershey & Pierce, 2015). Still to be determined are associations between hyperglycemia and peripheral neuropathy in patients with cancer with or without preexisting T2D.
Similar to pain, fatigue is common to cancer and T2D. Fatigue in individuals with poorly managed T2D who experience hyperglycemic events is thought to be a result of macro/microvascular complications from the interference with major organ function (Park et al., 2015; Singh & Kluding, 2013). In addition, physical and psychological distress related to a diabetes diagnosis can be associated with fatigue (Fritschi & Quinn, 2010; Young-Hyman et al., 2016). Cancer-related fatigue (CRF), however, may have multiple contributors. CRF is commonly reported as the most frequent and troubling symptom in patients with cancer, with 30%–60% reporting moderate to severe fatigue during treatment (Bower, 2014; Wright, Hammer, & D’Eramo Melkus, 2014). Dysregulation of proinflammatory cytokines related to the cancer and its treatments have been linked to CRF (Bower & Lamkin, 2012). Other biologic mechanisms associated with CRF include HPA axis dysfunction; circadian rhythm disruption; and regulation of serotonin, dopamine, and norepinephrine related to disturbances (Barsevick et al., 2013; Minton et al., 2013). A synergistic effect between cancer therapies and dopaminergic alterations from inflammatory responses has been shown to contribute to prolonged and devastating fatigue (Bower & Lamkin, 2012).
In alignment with these neuroendocrine and inflammatory triggers, as mentioned previously, genes that code for inflammatory markers have been associated with CRF (Wright et al., 2017). Taken together, factors that promote inflammation, such as hyperglycemia, may contribute to CRF. In addition, the dysregulation of the proinflammatory cytokine response from hyperglycemia and cancer may compound fatigue. Further research can provide a better understanding of the mechanistic similarities and differences between hyperglycemia and cancer related to fatigue.
In patients with T2D, hyperglycemia is a potential risk factor related to the development of depression in individuals with a genetic predisposition to affective disorders (Nagy et al., 2008). Among patients with T2D and cancer, evidence suggests that numerous factors are involved in depression and sleep disturbance, including single nucleotide polymorphisms in genes that code for inflammatory cytokine signaling molecules, psychological and physical stress responses through the HPA axis, high BMI, alterations in immune cell function, and interaction effects among depression, sleep disturbance, and stress (Bower & Lamkin, 2012; Doyle et al., 2013). In addition, sleep disturbances may trigger inflammatory pathways that can contribute to depression (Irwin, Olmstead, Ganz, & Haque, 2012). Sleep disturbance has also been found to be associated with increased risks for T2D (Yaggi, Araujo, & McKinlay, 2006), as well as insulin resistance and higher BG among patients with T2D (Ohkuma et al., 2014). The role of hyperglycemia in depression and sleep among patients with cancer has not been fully explored.
Implications for Research and Practice
Treating cancer is complex; therefore, it is essential to address factors that inhibit successful outcomes in patients undergoing treatment for cancer. Hyperglycemia is a modifiable risk factor, making it a desirable target of investigation. The evidence for hyperglycemia and T2D being risk factors for cancer has been established (Giovannucci et al., 2010). Regardless of T2D status, evidence suggests that hyperglycemia increases the risk for adverse events and outcomes in patients undergoing treatment for cancer. More in-depth investigations are needed to enhance the understanding of the metabolic links between hyperglycemia and the risk for cancer and adverse events and outcomes in patients undergoing treatment for cancer. In particular, the compounded effect of hyperglycemia- and cancer-related inflammation warrants further investigation. Various methods for measuring inflammatory biomarkers may help to further describe these pathways. For example, evaluating the neutrophil–lymphocyte ratio has been found to be informative for indicating the presence of inflammation (Cruz-Ramos et al., 2018; Dogan et al., 2018). Understanding the contributors to hyperglycemia can be used to develop focused screening tools to predict which individuals are at the greatest risk for hyperglycemic episodes and related adverse events and outcomes prior to the start of cancer therapies.
A number of challenges to conducting these studies exist, in part, because of a lack of consistencies in glycemic measurements and parameters (Storey, Von Ah, & Hammer, 2017). The following areas require further study:
• Genomic and epigenomic factors not described in the current review to get a more compre-hensive understanding of hyperglycemic–cancer–inflammatory pathways
• A therapeutic BG range, which may differ from the general population
• Glycemic screening guidelines prior to the start of cancer therapies
• Guidelines for glucose management and self-management during and following cancer therapies
Addressing these and other related areas can lead to mitigating the effect of hyperglycemia among patients with cancer. The long-term benefits are anticipated to include decreased adverse events and outcomes, improved survival, improved symptom management, and, ultimately, cancer prevention. The short-term goal is to improve overall health and quality of life for people with cancer.
Putting challenges aside, nurses can be proactive in guiding the management of glucose in patients with cancer. First, it is essential for nurses to understand the known and potential contributors to hyperglycemic events. Specifically, older age, high BMI, hyperglycemic-inducing chemotherapies, glucocorticoids, lack of physical activity, and stress should alert nurses to patients at risk for hyperglycemic events. Instituting protocols for increased glucose monitoring will capture hyperglycemic events earlier, which can then be expeditiously treated. Another challenge is that, even treated early, once a hyperglycemic event occurs, the deleterious physiologic response ensues. Until research can determine the glycemic risk profiles in patients with cancer and specific tailored guidelines for glucose regulation can be established in this population, being educated about the contribution of hyperglycemia to adverse events and outcomes is paramount for oncology nurses. 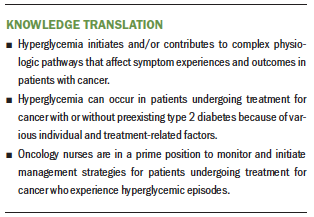
Conclusion
Hyperglycemia can occur in patients undergoing treatment for cancer regardless of their pretreatment glucose history. Understanding the complex pathways and identifying patients at greatest risk for hyperglycemic episodes can help in the development of preemptive interventions for improved patient outcomes. An important step is to identify the therapeutic range of glucose in people with cancer. Further research is needed to optimize care for this patient population.
About the Author(s)
Marilyn Hammer, PhD, DC, RN, FAAN, is the director of research and evidence-based practice at Mount Sinai Hospital and an associate professor in the Icahn School of Medicine at Mount Sinai in New York, NY; Susan Storey, PhD, RN, AOCNS®, is an assistant professor in the School of Nursing at Indiana University in Indianapolis; Denise Soltow Hershey, PhD, RN, FNP-BC, is an associate professor in the College of Nursing at Michigan State University in East Lansing; Veronica J. Brady, PhD, MSN, FNP-BC, BC-ADM, CDE, is an associate professor in the Reno School of Medicine at the University of Nevada; Ellen Davis, RN, MS, CDE, FAADE, is a consulting associate in the School of Nursing at Duke University in Durham, NC; Natalie Mandolfo, MSN, APRN-NP, AOCN®, is a PhD student in the College of Nursing at the University of Nebraska Medical Center in Omaha and a medical science liaison at Genentech in San Francisco, CA; Ashley Leak Bryant, PhD, RN-BC, OCN®, is an assistant professor at the University of North Carolina at Chapel Hill; and Jill Olausson, PhD, CDE, RN, is an assistant professor in the School of Nursing at Azusa Pacific University in California. Brady has previously consulted for the Oncology Dietitian Society and has previously served on speakers bureaus for RNsights. Hammer, Storey, Hershey, Davis, Mandolfo, and Bryant contributed to the conceptualization and design. Hammer, Hershey, Davis, and Mandolfo completed the data collection. Hammer, Davis, and Mandolfo provided statistical support and provided the analysis. All authors contributed to the manuscript preparation. Hammer can be reached at marilyn.hammer@mountsinai.org, with copy to ONFEditor@ons.org. (Submitted November 2018. Accepted January 2, 2019.)
References
Adeva-Andany, M.M., Pérez-Felpete, N., Fernández-Fernández, C., Donapetry-García, C., & Pazos-García, C. (2016). Liver glucose metabolism in humans. Bioscience Reports, 36, e00416. https://doi.org/10.1042/bsr20160385
Aikens, J.E. (2012). Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care, 35, 2472–2478. https://doi.org/10.2337/dc12-0181
American Diabetes Association. (2018a). Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care, 41(Suppl. 1), S13–S27. https://doi.org/10.2337/dc18-S002
American Diabetes Association. (2018b). Statistics about diabetes. Retrieved from http://www.diabetes.org/diabetes-basics/statistics
Anil, C., Akkurt, A., Ayturk, S., Kut, A., & Gursoy, A. (2013). Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metabolism, 62, 970–975. https://doi.org/10.1016/j.metabol.2013.01.009
Asgari, Y., Zabihinpour, Z., Salehzadeh-Yazdi, A., Schreiber, F., & Masoudi-Nejad, A. (2015). Alterations in cancer cell metabolism: The Warburg effect and metabolic adaptation. Genomics, 105, 275–281. https://doi.org/10.1016/j.ygeno.2015.03.001
Augustin, R. (2010). The protein family of glucose transport facilitators: It’s not only about glucose after all. International Union of Biochemisry and Molecular Biology Life, 62, 315–333. https://doi.org/10.1002/iub.315
Barsevick, A.M., Irwin, M.R., Hinds, P., Miller, A., Berger, A., Jacobsen, P., . . . Cella, D. (2013). Recommendations for high-priority research on cancer-related fatigue in children and adults. Journal of the National Cancer Institute, 105, 1432–1440. https://doi.org/10.1093/jnci/djt242
Barth, E., Albuszies, G., Baumgart, K., Matejovic, M., Wachter, U., Vogt, J., . . . Calzia, E. (2007). Glucose metabolism and catecholamines. Critical Care Medicine, 35(Suppl.), S508–S518. https://doi.org/10.1097/01.ccm.0000278047.06965.20
Barua, R., Templeton, A.J., Seruga, B., Ocana, A., Amir, E., & Ethier, J.L. (2018). Hyperglycaemia and survival in solid tumours: A systematic review and meta-analysis. Clinical Oncology Journal, 30, 215–224. https://doi.org/10.1016/j.clon.2018.01.003
Becker, S., Dossus, L., & Kaaks, R. (2009). Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Archives of Physiology and Biochemistry, 115, 86–96. https://doi.org/10.1080/13813450902878054
Beijers, A.J., Mols, F., & Vreugdenhil, G. (2014). A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Supportive Care in Cancer, 22, 1999–2007. https://doi.org/10.1007/s00520-014-2242-z
Biernacka, K.M., Uzoh, C.C., Zeng, L., Persad, R.A., Bahl, A., Gillatt, D., . . . Holly, J.M. (2013). Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocrine-Related Cancer, 20, 741–751. https://doi.org/10.1530/erc-13-0077
Bird, S.R., & Hawley, J.A. (2012). Exercise and type 2 diabetes: New prescription for an old problem. Maturitas, 72, 311–316. https://doi.org/10.1016/j.maturitas.2012.05.015
Bird, S.R., & Hawley, J.A. (2017). Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport and Exercise Medicine, 2, e000143. https://doi.org/10.1136/bmjsem-2016-000143
Bower, J.E. (2014). Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology, 11, 597–609. https://doi.org/10.1038/nrclinonc.2014.127
Bower, J.E., & Lamkin, D.M. (2012). Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain, Behavior, and Immunity, 30(Suppl.), S48–S57. https://doi.org/10.1016/j.bbi.2012.06.011
Brady, V., Thosani, S., Zhou, S., Bassett, R., Busaidy, N.L., & Lavis, V. (2014). Safe and effective dosing of basal-bolus insulin in patients receiving high-dose steroids for hyper-cyclophosphamide, doxorubicin, vincristine, and dexamethasone chemotherapy. Diabetes Technology and Theraputics, 16, 874–879. https://doi.org/10.1089/dia.2014.0115
Brownlee, M. (2005). The pathobiology of diabetic complications: A unifying mechanism. Diabetes, 54, 1615–1625.
Brunello, A., Kapoor, R., & Extermann, M. (2011). Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. American Journal of Clinical Oncology, 34, 292–296. https://doi.org/10.1097/COC.0b013e3181e1d0c0
Butler, S.O., Btaiche, I.F., & Alaniz, C. (2005). Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy, 25, 963–976.
Calle, M.C., & Fernandez, M.L. (2012). Inflammation and type 2 diabetes. Diabetes and Metabolism, 38, 183–191. https://doi.org/10.1016/j.diabet.2011.11.006
Clemmons, D.R. (2012). Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinology and Metabolism Clinics of North America, 41, 425–443, vii–viii. https://doi.org/10.1016/j.ecl.2012.04.017
Collier, B., Dossett, L.A., May, A.K., & Diaz, J.J. (2008). Glucose control and the inflammatory response. Nutrition in Clinical Practice, 23, 3–15.
Cruz-Ramos, M., Del Puerto-Nevado, L., Zheng, B., López-Bajo, R., Cebrian, A., Rodríguez-Remirez, M., . . . García-Foncillas, J. (2018). Prognostic significance of neutrophil-to lymphocyte ratio and platelet-to lymphocyte ratio in older patients with metastatic colorectal cancer. Journal of Geriatric Oncology. Advance online publication. https://doi.org/10.1016/j.jgo.2018.10.002
de Boer, M.C., Wörner, E.A., Verlaan, D., & van Leeuwen, P.A.M. (2017). The mechanisms and effects of physical activity on breast cancer. Clinical Breast Cancer, 17, 272–278. https://doi.org/10.1016/j.clbc.2017.01.006
de Mutsert, R., Sun, Q., Willett, W.C., Hu, F.B., & van Dam, R.M. (2014). Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: A cohort study. American Journal of Epidemiology, 179, 1353–1365. https://doi.org/10.1093/aje/kwu052
Derr, R.L., Hsiao, V.C., & Saudek, C.D. (2008). Antecedent hyperglycemia is associated with an increased risk of neutropenic infections during bone marrow transplantation. Diabetes Care, 31, 1972–1977. https://doi.org/10.2337/dc08-0574
Dogan, M., Algin, E., Guven, Z.T., Baykara, M., Kos, T.F., Bal, O., & Zengin, N. (2018). Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, neutrophil-platelet score and prognostic nutritional index: Do they have prognostic significance in metastatic pancreas cancer? Current Medical Research and Opinion, 34, 857–863. https://doi.org/10.1080/03007995.2017.1408579
Dombrowski, N.C., & Karounos, D.G. (2013). Pathophysiology and management strategies for hyperglycemia for patients with acute illness during and following a hospital stay. Metabolism, 62, 326–336. https://doi.org/10.1016/j.metabol.2012.07.020
Donovan, D. (2009). Management of peripheral neuropathy caused by microtubule inhibitors. Clinical Journal of Oncology Nursing, 13, 686–694. https://doi.org/10.1188/09.CJON.686-694
Doyle, T.A., de Groot, M., Harris, T., Schwartz, F., Strotmeyer, E.S., Johnson, K.C., & Kanaya, A. (2013). Diabetes, depressive symptoms, and inflammation in older adults: Results from the Health, Aging, and Body Composition Study. Journal of Psychosomatic Research, 75, 419–424. https://doi.org/10.1016/j.jpsychores.2013.08.006
Duan, W., Shen, X., Lei, J., Xu, Q., Yu, Y., Li, R., . . . Ma, Q. (2014). Hyperglycemia, a neglected factor during cancer progression. BioMed Research International, 2014, 461917. https://doi.org/10.1155/2014/461917
Esposito, K., Nappo, F., Marfella, R., Giugliano, G., Giugliano, F., Ciotola, M., . . . Giugliano, D. (2002). Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation, 106, 2067–2072.
Fisher, L., Hessler, D., Glasgow, R.E., Arean, P.A., Masharani, U., Naranjo, D., & Strycker, L.A. (2013). REDEEM: A pragmatic trial to reduce diabetes distress. Diabetes Care, 36, 2551–2558. https://doi.org/10.2337/dc12-2493
Fisher, L., Hessler, D.M., Polonsky, W.H., & Mullan, J. (2012). When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care, 35, 259–264. https://doi.org/10.2337/dc11-1572
Freund, A., Orjalo, A.V., Desprez, P.Y., & Campisi, J. (2010). Inflammatory networks during cellular senescence: Causes and consequences. Trends in Molecular Medicine, 16, 238–246. https://doi.org/10.1016/j.molmed.2010.03.003
Fritschi, C., & Quinn, L. (2010). Fatigue in patients with diabetes: A review. Journal of Psychosomatic Research, 69, 33–41. https://doi.org/10.1016/j.jpsychores.2010.01.021
Fuji, S., Kim, S.W., Mori, S., Fukuda, T., Kamiya, S., Yamasaki, S., . . . Takaue, Y. (2007). Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation, 84, 814–820.
Fuji, S., Löffler, J., Savani, B.N., Einsele, H., & Kapp, M. (2017). Hyperglycemia as a possible risk factor for mold infections—The potential preventative role of intensified glucose control in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation, 52, 657–662. https://doi.org/10.1038/bmt.2016.306
Gale, S.C., Sicoutris, C., Reilly, P.M., Schwab, C.W., & Gracias, V.H. (2007). Poor glycemic control is associated with increased mortality in critically ill trauma patients. American Surgeon, 73, 454–460.
Germenis, A.E., & Karanikas, V. (2007). Immunoepigenetics: The unseen side of cancer immunoediting. Immunology and Cell Biology, 85, 55–59. https://doi.org/10.1038/sj.icb.7100006
Giacco, F., & Brownlee, M. (2010). Oxidative stress and diabetic complications. Circulation Research, 107, 1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545
Giovannucci, E., Harlan, D.M., Archer, M.C., Bergenstal, R.M., Gapstur, S.M., Habel, L.A., . . . Yee, D. (2010). Diabetes and cancer: A consensus report. Diabetes Care, 33, 1674–1685. https://doi.org/10.2337/dc10-0666
Greene, E.R., Huang, S., Serhan, C.N., & Panigrahy, D. (2011). Regulation of inflammation in cancer by eicosanoids. Prostaglandins and Other Lipid Mediators, 96, 27–36. https://doi.org/10.1016/j.prostaglandins.2011.08.004
Hammer, M.J., Casper, C., Gooley, T.A., O’Donnell, P.V., Boeckh, M., & Hirsch, I.B. (2009). The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biology of Blood and Marrow Transplantation, 15, 344–351. https://doi.org/10.1016/j.bbmt.2008.12.488
Hammer, M.J., D’Eramo Melkus, G., Knobf, M.T., Casper, C., Fletcher, J., & Cleland, C.M. (2016). Glycemic status and infection risk in nondiabetic autologous hematopoietic cell transplantation recipients. Biological Research for Nursing, 18, 344–350. https://doi.org/10.1177/1099800415619227
Hardman, W.E. (2014). Diet components can suppress inflammation and reduce cancer risk. Nutrition Research and Practice, 8, 233–240. https://doi.org/10.4162/nrp.2014.8.3.233
Herkenne, S., Paques, C., Nivelles, O., Lion, M., Bajou, K., Pollenus, T., . . . Struman, I. (2015). The interaction of uPAR with VEGFR2 promotes VEGF-induced angiogenesis. Science Signaling, 8, ra117. https://doi.org/10.1126/scisignal.aaa2403
Hershey, D.S., Bryant, A.L., Olausson, J., Davis, E.D., Brady, V.J., & Hammer, M. (2014). Hyperglycemic-inducing neoadjuvant agents used in treatment of solid tumors: A review of the literature [Online exclusive]. Oncology Nursing Forum, 41, E343–E354. https://doi.org/10.1188/14.onf.e343-e354
Hershey, D.S., & Pierce, S.J. (2015). Examining patterns of multivariate, longitudinal symptom experiences among older adults with type 2 diabetes and cancer via cluster analysis. European Journal of Oncology Nursing, 19, 716–723. https://doi.org/10.1016/j.ejon.2015.05.006
Holmes, B.E. (1940). Inhibition by fluoride of glucose breakdown in tumour tissue and retina extracts. Biochemical Journal, 34, 926–930.
Irwin, M.R., Olmstead, R.E., Ganz, P.A., & Haque, R. (2012). Sleep disturbance, inflammation and depression risk in cancer survivors. Brain, Behavior, and Immunity, 30(Suppl.), S58-S67. https://doi.org/10.1016/j.bbi.2012.05.002
Kalsbeek, A., Bruinstroop, E., Yi, C.X., Klieverik, L.P., La Fleur, S.E., & Fliers, E. (2010). Hypothalamic control of energy metabolism via the autonomic nervous system. Annals of the New York Academy of Sciences, 1212, 114–29. https://doi.org/10.1111/j.1749-6632.2010.05800.x.
Kellenberger, L.D., Bruin, J.E., Greenaway, J., Campbell, N.E., Moorehead, R.A., Holloway, A.C., & Petrik, J. (2010). The role of dysregulated glucose metabolism in epithelial ovarian cancer. Journal of Oncology, 2010, 514310. https://doi.org/10.1155/2010/514310
Klaunig, J.E., & Kamendulis, L.M. (2004). The role of oxidative stress in carcinogenesis. Annual Review of Pharmacology and Toxicology, 44, 239–267. https://doi.org/10.1146/annurev.pharmtox.44.101802.121851
Kristensen, T., Fredholm, M., & Cirera, S. (2015). Expression study of GLUT4 translocation-related genes in a porcine pre-diabetic model. Mammalian Genome, 26, 650–657. https://doi.org/10.1007/s00335-015-9601-z
Kumar, V., & Gabrilovich, D.I. (2014). Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology, 143, 512–519. https://doi.org/10.1111/imm.12380
Lasselin, J., & Capuron, L. (2014). Chronic low-grade inflammation in metabolic disorders: Relevance for behavioral symptoms. Neuroimmunomodulation, 21, 95–101. https://doi.org/10.1159/000356535
Li, H., Batth, I.S., Qu, X., Xu, L., Song, N., Wang, R., & Liu, Y. (2017). IGF-IR signaling in epithelial to mesenchymal transition and targeting IGF-IR therapy: Overview and new insights. Molecular Cancer, 16, 6. https://doi.org/10.1186/s12943-016-0576-5
Li, W., Ma, Q., Liu, J., Han, L., Ma, G., Liu, H., . . . Wu, E. (2012). Hyperglycemia as a mechanism of pancreatic cancer metastasis. Frontiers in Bioscience, 17, 1761–1774.
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gotzsche, P.C., Ioannidis, J.P., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
Manoochehri Khoshinani, H., Afshar, S., & Najafi, R. (2016). Hypoxia: A double-edged sword in cancer therapy. Cancer Investigation, 34, 536–545. https://doi.org/10.1080/07357907.2016.1245317
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454, 436–444. https://doi.org/10.1038/nature07205
Massa, M.L., Gagliardino, J.J., & Francini, F. (2011). Liver glucokinase: An overview on the regulatory mechanisms of its activity. International Union of Biochemisry and Molecular Biology Life, 63, 1–6. https://doi.org/10.1002/iub.411
Miaskowski, C. (2016). Future directions in symptom cluster research. Seminars in Oncology Nursing, 32, 405–415. https://doi.org/10.1016/j.soncn.2016.08.006
Miaskowski, C., Cooper, B.A., Aouizerat, B., Melisko, M., Chen, L.M., Dunn, L., . . . Kearney, N. (2017). The symptom phenotype of oncology outpatients remains relatively stable from prior to through 1 week following chemotherapy. European Journal of Cancer Care, 26, e12437. https://doi.org/10.1111/ecc.12437
Miaskowski, C., Cooper, B.A., Paul, S.M., Dodd, M., Lee, K., Aouizerat, B.E., . . . Bank, A. (2006). Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis [Online exclusive]. Oncology Nursing Forum, 33, E79–E89. https://doi.org/10.1188/06.ONF.E79-E89
Minton, O., Berger, A., Barsevick, A., Cramp, F., Goedendorp, M., Mitchell, S.A., & Stone, P.C. (2013). Cancer-related fatigue and its impact on functioning. Cancer, 119(Suppl. 11), 2124–2130. https://doi.org/10.1002/cncr.28058
Nagy, G., Ronai, Z., Somogyi, A., Sasvari-Szekely, M., Rahman, O.A., Mate, A., . . . Nemoda, Z. (2008). P2RX7 Gln460Arg polymorphism is associated with depression among diabetic patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 32, 1884–1888. https://doi.org/10.1016/j.pnpbp.2008.08.021
Ohkuma, T., Fujii, H., Iwase, M., Ogata-Kaizu, S., Ide, H., Kikuchi, Y., . . . Kitazono, T. (2014). U-shaped association of sleep duration with metabolic syndrome and insulin resistance in patients with type 2 diabetes: The Fukuoka Diabetes Registry. Metabolism, 63, 484–491. https://doi.org/10.1016/j.metabol.2013.12.001
Pachman, D.R., Qin, R., Seisler, D., Smith, E.M., Kaggal, S., Novotny, P., . . . Loprinzi, C.L. (2016). Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Supportive Care in Cancer, 24, 5059–5068. https://doi.org/10.1007/s00520-016-3373-1
Park, S.E., Park, C.Y., & Sweeney, G. (2015). Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Critical Reviews in Clinical Laboratory Sciences, 52, 180–190. https://doi.org/10.3109/10408363.2015.1023429
Pud, D., Ben Ami, S., Cooper, B.A., Aouizerat, B.E., Cohen, D., Radiano, R., . . . Miaskowski, C. (2008). The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. Journal of Pain and Symptom Management, 35, 162–170. https://doi.org/10.1016/j.jpainsymman.2007.03.010
Roder, P.V., Wu, B., Liu, Y., & Han, W. (2016). Pancreatic regulation of glucose homeostasis. Experimental and Molecular Medicine, 48, e219. https://doi.org/10.1038/emm.2016.6
Rodríguez-Hernández, H., Simental-Mendía, L.E., Rodríguez-Ramírez, G., & Reyes-Romero, M.A. (2013). Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. International Journal of Endocrinology, 2013, 678159. https://doi.org/10.1155/2013/678159
San-Millán, I., & Brooks, G.A. (2016). Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis, 38, 119–133. https://doi.org/10.1093/carcin/bgw127
Scott, R.B. (1968). Glycogen in human peripheral blood leukocytes. I. Characteristics of the synthesis and turnover of glycogen in vitro. Journal of Clinical Investigation, 47, 344–352. https://doi.org/10.1172/jci105730
Scott, R.B., & Still, WJ. (1968). Glycogen in human peripheral blood leukocytes. II. The macromolecular state of leukocyte glycogen. Journal of Clinical Investigation, 47, 353–359. https://doi.org/10.1172/jci105731
Singh, R., & Kluding, P.M. (2013). Fatigue and related factors in people with type 2 diabetes. Diabetes Educator, 39, 320–326. https://doi.org/10.1177/0145721713479144
Smiley, D., & Umpierrez, G.E. (2010). Management of hyperglycemia in hospitalized patients. Annals of the New York Academy of Sciences, 1212, 1–11. https://doi.org/10.1111/j.1749-6632.2010.05805.x
Snoek, F.J., Bremmer, M.A., & Hermanns, N. (2015). Constructs of depression and distress in diabetes: Time for an appraisal. Lancet Diabetes Endocrinology, 3, 450–460. https://doi.org/10.1016/s2213-8587(15)00135-7
Stjernholm, R.L. (1967). Carbohydrate metabolism in leukocytes. VII. Metabolism of glucose, acetate, and propionate by human plasma cells. Journal of Bacteriology, 93, 1657–1661.
Storey, S., & Von Ah, D. (2015). Prevalence and impact of hyperglycemia on hospitalized leukemia patients. European Journal of Oncology Nursing, 19, 13–17. https://doi.org/10.1016/j.ejon.2014.08.005
Storey, S., & Von Ah, D. (2016). Impact of hyperglycemia and age on outcomes in patients with acute myeloid leukemia. Oncology Nursing Forum, 43, 595–601. https://doi.org/10.1188/16.ONF.595-601
Storey S., Von Ah, D., & Hammer, M.J. (2017). Measurement of hyperglycemia and impact on health outcomes in people with cancer: Challenges and opportunities [Online exclusive]. Oncology Nursing Forum, 44, E141–E151. https://doi.org/10.1188/17.ONF.E141-E151
Tavakoli, M., Mojaddidi, M., Fadavi, H., & Malik, R.A. (2008). Pathophysiology and treatment of painful diabetic neuropathy. Current Pain and Headache Reports, 12, 192–197.
Testa, J.R., & Tsichlis, P.N. (2005). AKT signaling in normal and malignant cells. Oncogene, 24, 7391–7393. https://doi.org/10.1038/sj.onc.1209100
Teunissen, S.C., de Graeff, A., de Haes, H.C., & Voest, E.E. (2006). Prognostic significance of symptoms of hospitalised advanced cancer patients. European Journal of Cancer, 42, 2510–2516. https://doi.org/10.1016/j.ejca.2006.05.025
Turner, N., & Grose, R. (2010). Fibroblast growth factor signalling: From development to cancer. Nature Reviews Cancer, 10, 116–129. https://doi.org/10.1038/nrc2780
Uebelhoer, M., & Iruela-Arispe, M.L. (2016). Cross-talk between signaling and metabolism in the vasculature. Vascular Pharmacology, 83, 4–9. https://doi.org/10.1016/j.vph.2016.06.002
Underwood, J.M., Townsend, J.S., Stewart, S.L., Buchannan, N., Ekwueme, D.U., Hawkins, N.A., . . . Fairley, T.L. (2012). Surveillance of demographic characteristics and health behaviors among adult cancer survivors—Behavioral Risk Factor Surveillance System, United States, 2009. Morbidity and Mortality Weekly Report Surveillance Summaries, 6, 1–23.
Villarreal-Garza, C., Shaw-Dulin, R., Lara-Medina, F., Bacon, L., Rivera, D., Urzua, L., . . . Herrera, L.A. (2012). Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Experimental Diabetes Research, 2012, 732027. https://doi.org/10.1155/2012/732027
Wang, Y., Wang, W., Sanidad, K.Z., Shih, P.A., Zhao, X., & Zhang, G. (2018). Eicosanoid signaling in carcinogenesis of colorectal cancer. Cancer Metastasis Review, 37, 257–267. https://doi.org/10.1007/s10555-018-9739-8
Werner, H., Sarfstein, R., LeRoith, D., & Bruchim, I. (2016). Insulin-like growth factor 1 signaling axis meets p53 genome protection pathways. Frontiers in Oncology, 6, 159. https://doi.org/10.3389/fonc.2016.00159
Werner, H., Weinstein, D., & Bentov, I. (2008). Similarities and differences between insulin and IGF-I: Structures, receptors, and signalling pathways. Archives of Physiology and Biochemistry, 114, 17–22. https://doi.org/10.1080/13813450801900694
Wright, F., Hammer, M., Paul, S.M., Aouizerat, B.E., Kober, K.M., Conley, Y.P., . . . Miaskowski, C. (2017). Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine, 91, 187–210. https://doi.org/10.1016/j.cyto.2016.12.023
Wright, F., Hammer, M.J., & D’Eramo Melkus, G. (2014). Associations between multiple chronic conditions and cancer-related fatigue: An integrative review. Oncology Nursing Forum, 41, 399–410. https://doi.org/10.1188/14.onf.41-04ap
Wright, J.L., Plymate, S.R., Porter, M.P., Gore, J.L., Lin, D.W., Hu, E., & Zeliadt, S.B. (2013). Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer and Prostatic Disease, 16, 204–208. https://doi.org/10.1038/pcan.2013.5
Yaggi, H.K., Araujo, A.B., & McKinlay, J.B. (2006). Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care, 29, 657–661.
Yeluri, S., Madhok, B., Prasad, K.R., Quirke, P., & Jayne, D.G. (2009). Cancer’s craving for sugar: An opportunity for clinical exploitation. Journal of Cancer Research and Clinical Oncology, 135, 867–877. https://doi.org/10.1007/s00432-009-0590-8
Young-Hyman, D., de Groot, M., Hill-Briggs, F., Gonzalez, J.S., Hood, K., & Peyrot, M. (2016). Psychosocial care for people with diabetes: A position statement of the American Diabetes Association. Diabetes Care, 39, 2126–2140. https://doi.org/10.2337/dc16-2053
Yu, T., Jhun, B.S., & Yoon, Y. (2011). High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxidants and Redox Signaling, 14, 425–437. https://doi.org/10.1089/ars.2010.3284
Zafar, U., Khaliq, S., Ahmad, H.U., Manzoor, S., & Lone, K.P. (2018). Metabolic syndrome: An update on diagnostic criteria, pathogenesis, and genetic links. Hormones, 17, 299–313. https://doi.org/10.1007/s42000-018-0051-3
Zeng, L., Biernacka, K.M., Holly, J.M., Jarrett, C., Morrison, A.A., Morgan, A., . . . Perks, C.M. (2010). Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: The role of fatty acid synthase. Endocrine-Related Cancer, 17, 539–551. https://doi.org/10.1677/erc-09-0221
Zheng, C., Beresford, S.A., Van Horn, L., Tinker, L.F., Thomson, C.A., Neuhouser, M.L., . . . Prentice, R.L. (2014). Simultaneous association of total energy consumption and activity-related energy expenditure with risks of cardiovascular disease, cancer, and diabetes among postmenopausal women. American Journal of Epidemiology, 180, 526–535. https://doi.org/10.1093/aje/kwu152
Zhou, D., Shao, L., & Spitz, D.R. (2014). Reactive oxygen species in normal and tumor stem cells. Advances in Cancer Research, 122, 1–67. https://doi.org/10.1016/b978-0-12-420117-0.00001-3
Zhou, X.H., Qiao, Q., Zethelius, B., Pyörälä, K., Söderberg, S., Pajak, A., . . . Tuomilehto, J. (2010). Diabetes, prediabetes and cancer mortality. Diabetologia, 53, 1867–1876. https://doi.org/10.1007/s00125-010-1796-7
Zhou, Y., Zhang, X., Gu, C., & Xia, J. (2015). Influence of diabetes mellitus on mortality in breast cancer patients. ANZ Journal of Surgery, 85, 972–978. https://doi.org/10.1111/ans.12877
Ziech, D., Franco, R., Georgakilas, A.G., Georgakila, S., Malamou-Mitsi, V., Schoneveld, O., . . . Panayiotidis, M. I. (2010). The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chemico-Biological Interactions, 188, 334–339. https://doi.org/10.1016/j.cbi.2010.07.010


