Effects of Home-Based Exercise Training for Patients With Lung Cancer
Problem Identification: To investigate the effectiveness of home-based exercise training on exercise capacity, dyspnea, anxiety, depression, and health-related quality of life (HRQOL).
Literature Search: A systematic literature search of the Cochrane Central Register of Randomized Controlled Trials, Embase®, PubMed®, and Web of Science databases was performed for articles published through July 22, 2018.
Data Evaluation: The meta-analysis was conducted with Review Manager, version 5.3, following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Synthesis: 10 articles with a total of 453 patients met the inclusion criteria. Home-based exercise training was found to increase the six-minute walk distance. In addition, anxiety was also improved after the intervention. However, no improvements in dyspnea, depression, or HRQOL were observed.
Implications for Research: Home-based exercise training as a nursing intervention for promoting the rehabilitation of patients with lung cancer can be recommended, but more research should be undertaken to determine the most effective exercises and follow-up methods.
Jump to a section
Lung cancer is one of the most common malignancies, with about 1.6 million new cases of lung cancer diagnosed worldwide each year, which is predicted to increase to 2.2 million cases by 2020 (Hong et al., 2015; Siegel, Miller, & Jemal, 2018). Surgery is usually the preferred treatment for patients with early-stage disease (stages I, II, and IIIA), which is often staged using the TNM (tumor, nodes, metastasis) classification system (Goldstraw et al., 2016). However, surgery is deemed suitable for only 25% of patients because of advanced disease or dysfunction, and others choose radiation therapy or chemotherapy (Brunelli et al., 2009). Although these treatments prevent cancer cells from spreading, they also cause a higher burden of symptoms for patients (Cleeland et al., 2013). The most common respiratory symptom in patients with lung cancer is dyspnea, which limits exercise capacity (Lou et al., 2017). In addition, anxiety and depression are the most common psychological problems in patients with lung cancer, with rates of about 21% and 39%, respectively (Jung et al., 2018). Dyspnea, negative emotions, and decreased physical activity may all be potential causes of impaired health-related quality of life (HRQOL), which is closely related to poor prognosis and a low survival rate in patients with cancer (Giese-Davis et al., 2011).
Exercise training is one of the key components of current management of lung cancer and plays a vital role in the rehabilitation of patients. There are many types of exercise training, including endurance training, interval training, strength training, and respiratory muscle training. The most common forms of endurance training are cycling and walking (Spruit et al., 2013). Interval training is an alternative form of endurance training in which high-intensity exercise is often interspersed with rest or lower-intensity exercise. Strength training is a way of training a local muscle group by repeatedly lifting a relatively heavy load (O’Shea, Taylor, & Paratz, 2009). Respiratory muscle training aims to correct the patient’s abnormal breathing pattern and improve respiratory muscle function (Nici et al., 2006).
Exercise training has been shown to improve dyspnea, exercise capacity, and HRQOL during hospitalization (Lai et al., 2017; Mujovic et al., 2014). However, these effects decline over time when patients discontinue exercise training after discharge (Spruit et al., 2013). In addition, for patients undergoing radiation therapy and chemotherapy, going to the outpatient department for exercise training can be limited by weather, transportation issues, and illness (Temel et al., 2009). Home-based exercise training might overcome some of these barriers and target a broader range of patients with lung cancer who would benefit from exercise training.
The evidence for effective home-based exercise training for patients with lung cancer is limited by small sample sizes and differences in study designs (Chen, Tsai, Wu, Lin, & Lin, 2015; Coats et al., 2013; Hoffman et al., 2014). Consequently, the current authors conducted a systematic review and meta-analysis to investigate the effects of home-based exercise training on exercise capacity, dyspnea, anxiety, depression, and HRQOL among patients with lung cancer.
Methods
The review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Liberati et al., 2009).
Eligibility Criteria
Randomized controlled trials (RCTs) and non-RCTs were considered for inclusion if they had the following characteristics:
• Involved patients diagnosed with lung cancer or patients diagnosed with lung cancer among a mixed cancer cohort
• Defined home-based exercise as conducting aerobic training, resistance training, or a combination of both at home and including regular follow-up via home visit, telephone, or logbook
• Reported at least one of the following outcomes: exercise capacity assessed with six-minute walk distance (6MWD); dyspnea assessed with the Borg scale; anxiety and depression assessed with the Hospital Anxiety and Depression Scale (HADS); and HRQOL assessed with the European Organisation for Research and Treatment of Cancer QOL Questionnaire–Core 30 (EORTC QLQ-C30)
• Was a full-text article published in English in peer-reviewed journal
Search Strategy
An electronic literature search was performed using four databases: the Cochrane Central Register of Randomized Controlled Trials, Embase®, PubMed®, and Web of Science. All databases were searched for relevant articles published from inception to July 22, 2018. A hand search for references included in each article was also performed to identify additional articles.
Data Collection
Two of the current authors (Y.-Q.W. and X.L.) screened all titles and abstracts to identify all potentially eligible articles that met the inclusion criteria. Additional full-text analysis was independently performed by two reviewers (Y.-Y.Y. and R.-C.M.) to determine the articles’ eligibility for data extraction. The following information was recorded:
• Publication data (year of publication, first author, study design, location)
• Demographic data (sample size; number of intervention and control groups; patient type and stage of cancer, treatment method, age, gender, and body mass index)
• Study data (intervention methods, frequency, length of intervention, approaches to follow-up)
• Outcome measures
A data extraction sheet was used to extract data, and discrepancies were resolved by discussion.
Risk of Bias in Individual Studies
Risk of bias was assessed using the Cochrane risk of bias tool (Higgins & Green, 2011) for RCTs and the methodological index for nonrandomized studies (MINORS) (Slim et al., 2003) for non-RCTs. The Cochrane risk of bias tool assesses seven areas of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting bias, and other biases. Each item was rated as having a low, unclear, or high risk of bias. MINORS consists of 12 items that assess specific methodologic criteria, of which 8 are for noncomparative studies and 4 are for comparative studies. A score of 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate) was given for each item on the MINORS checklist, resulting in a maximum score of 16 for noncomparative studies and 24 for comparative studies (Slim et al., 2003). Two of the current authors (Y.-Q.W. and X.L.) independently evaluated the quality of the included studies, and disagreements were settled through discussion.
Statistical Analysis
Statistical analysis was conducted using Review Manager, version 5.3. Continuous outcomes were counted for the mean difference (MD) with a 95% confidence interval (CI). Forest plots were constructed to illustrate the study-specific effect size. A two-sided p value of less than 0.05 was considered statistically significant. Statistical heterogeneity among the studies was assessed using Cochran Q tests and I2 tests. Given the diversity of the interventions and the observed statistical heterogeneity (I2 > 50%), random effects models were used to pool the effect sizes. Otherwise, a fixed effects model was used.
A sensitivity analysis was conducted to evaluate whether findings were stable and whether they were driven by a single study. The merged results before the changes and the adjusted results were compared to determine the sources of heterogeneity.
Results
Study Selection
The original electronic search identified 695 articles: 52 from the Cochrane Central Register of Randomized Controlled Trials, 52 from Embase, 190 from PubMed, and 401 from Web of Science. Duplicates were removed, and 530 articles remained. After further review of the title and abstract, 492 additional articles were excluded because they did not meet the inclusion criteria. A total of 38 articles were retained for full-text analysis, and 10 were ultimately selected for inclusion in the meta-analysis (Andersen, Vinther, Poulsen, & Mellemgaard, 2011, 2013; Arbane, Tropman, Jackson, & Garrod, 2011; Brocki et al., 2014; Chen et al., 2015; Coats et al., 2013; Granger, Chao, McDonald, Berney, & Denehy, 2013; Hoffman et al., 2014; Olivier et al., 2018; Quist et al., 2012). A summary of the literature selection process is shown in Figure 1. 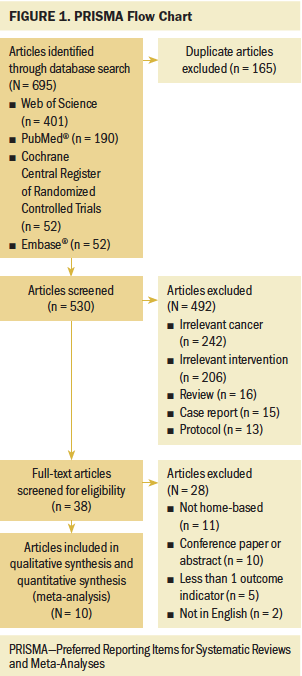
Study Characteristics
The main characteristics of the included studies are summarized in Table 1. 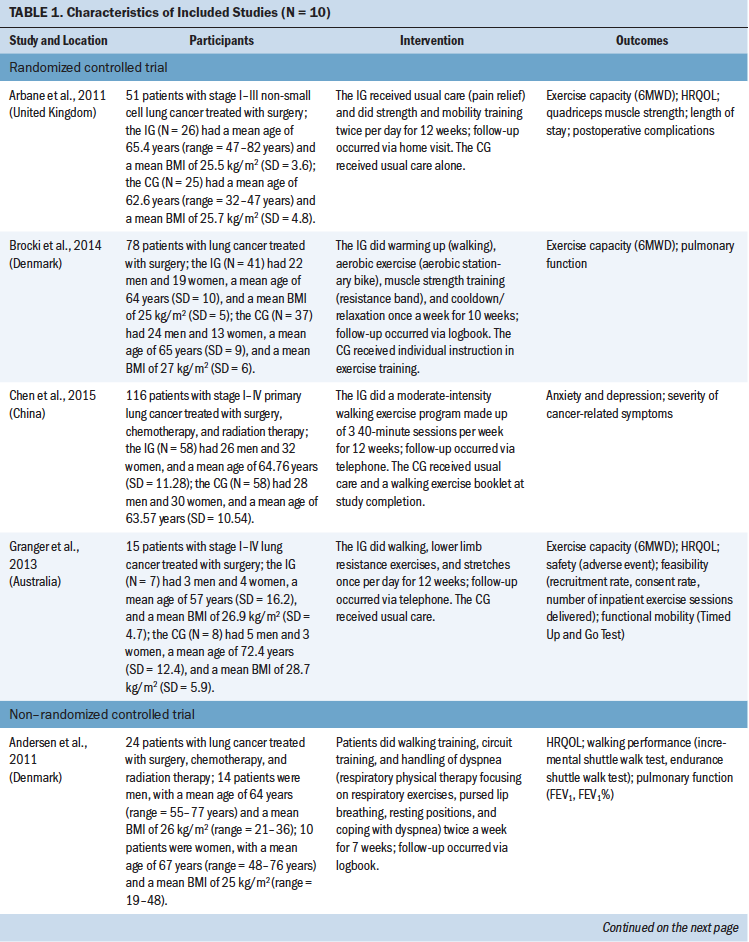
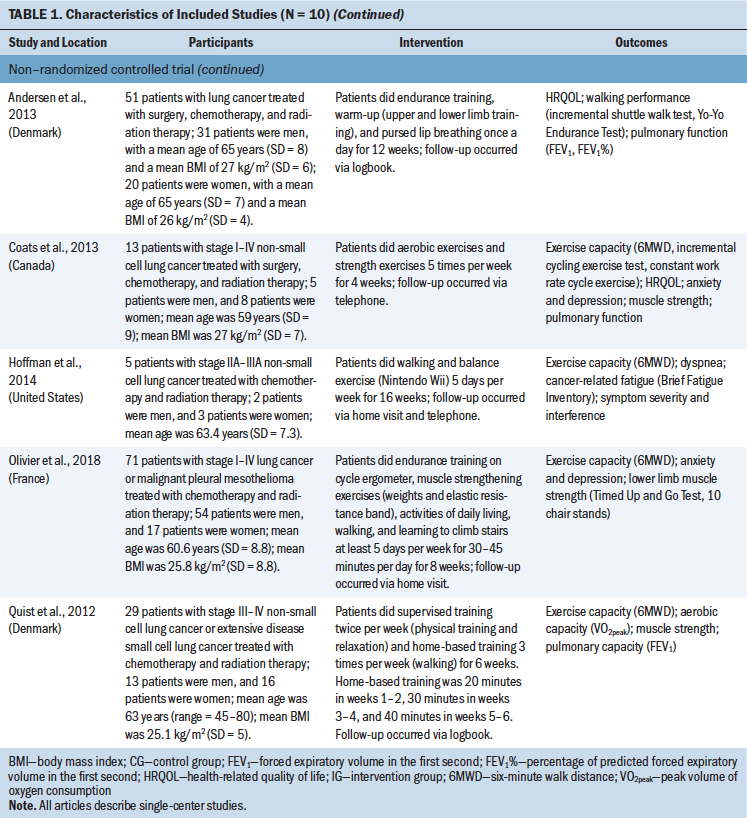
Design: The systematic review included four RCTs (Arbane et al., 2011; Brocki et al., 2014; Chen et al., 2015; Granger et al., 2013) and six non-RCTs (Andersen et al., 2011, 2013; Coats et al., 2013; Hoffman et al., 2014; Olivier et al., 2018; Quist et al., 2012). All 10 articles described single-center studies and included data from the United States, China, the United Kingdom, Australia, France, Canada, and Denmark.
Participants: A total of 453 eligible patients were included in this systematic review. Most studies included patients with lung cancer, and only one study (Olivier et al., 2018) included patients with lung cancer or malignant pleural mesothelioma. The sample size ranged from 5 to 116 patients, and patient age ranged from 48 to 77 years. Four studies (Chen et al., 2015; Coats et al., 2013; Granger et al., 2013; Olivier et al., 2018) included patients with stage I–IV disease. Arbane et al. (2011) included patients with stage I–III disease; Hoffman et al. (2014) included patients with stage IIA–IIIA disease; and Quist et al. (2012) included patients with stage III–IV disease. Three studies (Andersen et al., 2011, 2013; Brocki et al., 2014) did not report lung cancer stage. In three studies (Arbane et al., 2011; Brocki et al., 2014; Granger et al., 2013), the treatment approach was surgery. The combination of chemotherapy and radiation therapy was used in three studies (Hoffman et al., 2014; Olivier et al., 2018; Quist et al., 2012), whereas the combination of surgery, chemotherapy, and radiation therapy was used in four studies (Andersen et al., 2011, 2013; Chen et al., 2015; Coats et al., 2013).
Type of intervention: The home-based exercise training consisted of aerobic training, resistance training, and breathing exercises. The total length of the interventions ranged from 4 to 16 weeks, and each exercise had a different frequency. Follow-up was via telephone (Chen et al., 2015; Coats et al., 2013; Granger et al., 2013); home visit (Arbane et al., 2011; Olivier et al., 2018); or logbook (Andersen et al., 2011, 2013; Brocki et al., 2014; Quist et al., 2012). One study (Hoffman et al., 2014) conducted follow-up via home visit and telephone.
Outcomes: The primary outcomes were exercise capacity and dyspnea. Seven studies (Arbane et al., 2011; Brocki et al., 2014; Coats et al., 2013; Granger et al., 2013; Hoffman et al., 2014; Olivier et al., 2018; Quist et al., 2012) reported exercise capacity, which was measured using the 6MWD, a simple submaximal exercise test that measures the distance walked on a hard, level surface in six minutes to assess exercise capacity and has strong test-retest reliability (intraclass correlation = 0.97) (Hamilton & Haennel, 2000; Yang et al., 2018). Three studies (Brocki et al., 2014; Coats et al., 2013; Hoffman et al., 2014) focused on continuous data for dyspnea, which were measured using the Borg scale. The Borg scale measures dyspnea on a scale ranging from 0 to 20, with higher scores indicating greater dyspnea severity (Borg, 1974).
The secondary outcomes were anxiety and depression, which were measured using HADS, a reliable and widely used tool to assess anxiety and depression in patients with cancer (Duijts et al., 2012), and HRQOL, which was measured using the EORTC QLQ-C30, a valid and reliable tool to assess the HRQOL of patients with cancer. Each of the seven items on the HADS anxiety subscale and on the HADS depression subscale was scored on a four-point scale ranging from 0 (not at all) to 3 (very much so), with potential total subscale scores ranging from 0 to 21. Higher scores indicate higher levels of anxiety or depression (Zigmond & Snaith, 1983). The EORTC QLQ-C30 consists of five functional domains (physical, emotional, social, role, and cognitive); three symptom domains (pain, fatigue, and nausea and vomiting); six single items (appetite loss, dyspnea, diarrhea, constipation, insomnia, and financial difficulties); and global health status (Granger, McDonald, Berney, Chao, & Denehy, 2011). The EORTC QLQ-C30 is scored on a scale ranging from 0 to 100. For the five functional domains and global health status, 0 indicates the lowest level of function (worst score) and 100 indicates the highest level of function (best score); for the remaining items, 0 indicates the lowest level of symptoms (best score) and 100 indicates the highest level of symptoms (worst score).
Three studies (Chen et al., 2015; Coats et al., 2013; Olivier et al., 2018) reported anxiety and depression. Five studies (Andersen et al., 2011, 2013; Arbane et al., 2011; Coats et al., 2013; Granger et al., 2013) reported HRQOL, but Arbane et al. (2011) reported only global health status, and Granger et al. (2013) did not report insomnia, appetite loss, constipation, diarrhea, or financial difficulties.
Quality Assessment
Using the Cochrane risk of bias tool, the current authors classified the four RCTs as having random sequence generation for the enrolled patients and as having allocation concealment. All RCTs were also found not to have other biases. In addition, one study was found to perform adequate blinding of participants and personnel (Granger et al., 2013); three studies performed adequate blinding of the outcome assessment (Arbane et al., 2011; Brocki et al., 2014; Granger et al., 2013); three studies had complete outcome data (Brocki et al., 2014; Chen et al., 2015; Granger et al., 2013); and three studies did not contain selective reporting bias (Arbane et al., 2011; Brocki et al., 2014; Granger et al., 2013.
Using the MINORS checklist, the current authors determined that the six non-RCTs had clearly stated aims, collected data prospectively, included consecutive patients, and had a follow-up period appropriate to the aim. However, all studies lacked the reporting of an unbiased assessment of study endpoint and did not include prospective calculation of the study size.
Overall Analysis
Table 2 provides the effects of the intervention on exercise capacity, dyspnea, anxiety, and depression. Table 3 provides the effects of the intervention on HRQOL. 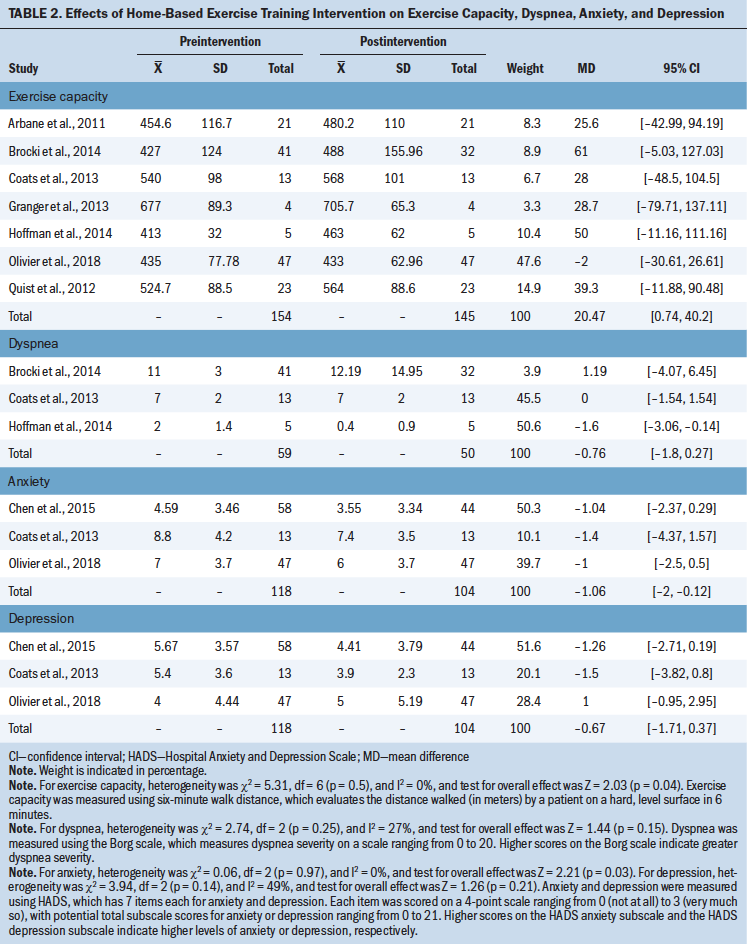
Exercise capacity: Seven studies (Arbane et al., 2011; Brocki et al., 2014; Coats et al., 2013; Granger et al., 2013; Hoffman et al., 2014; Olivier et al., 2018; Quist et al., 2012) assessed the effect of the intervention on exercise capacity using the 6MWD. There was no heterogeneity in the 6MWD (I2 = 0%), so the fixed effects model was selected for this analysis. Results showed that home-based exercise training could increase the 6MWD by an average of 20.47 m (MD = 20.47; 95% CI [0.74, 40.2]; p = 0.04). 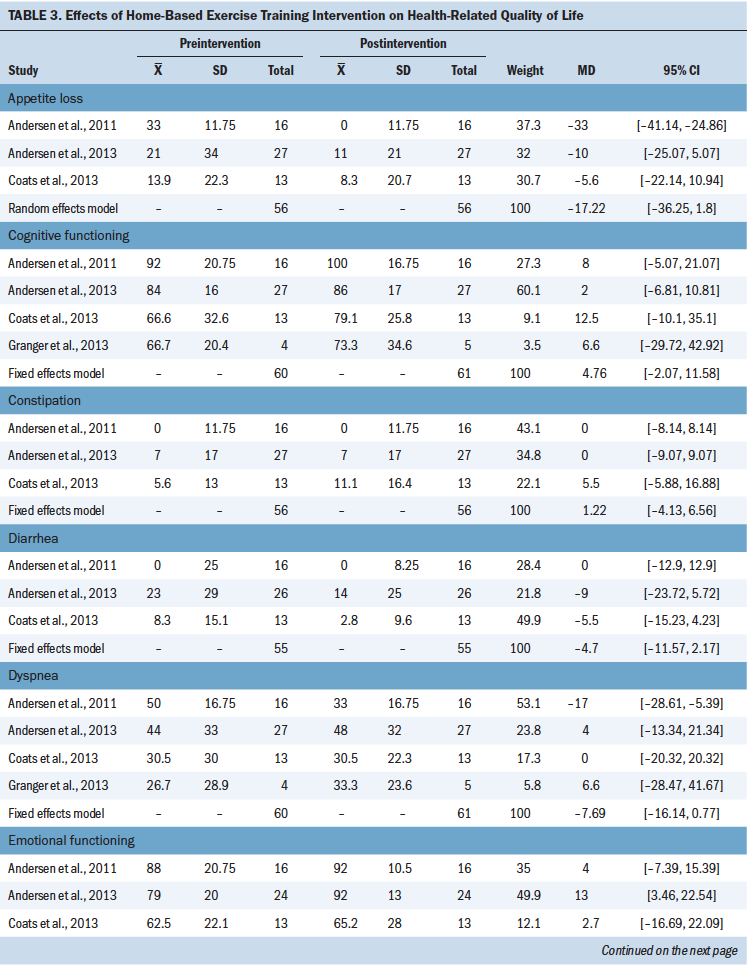
[[{"fid":"51241","view_mode":"default","fields":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":false,"field_file_image_title_text[und][0][value]":false},"link_text":null,"type":"media","field_deltas":{"1":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":false,"field_file_image_title_text[und][0][value]":false}},"attributes":{"class":"media-element file-default","data-delta":"1"}}]]
Dyspnea: Three studies (Brocki et al., 2014; Coats et al., 2013; Hoffman et al., 2014) reported the effect of the intervention on dyspnea. There was low heterogeneity in dyspnea (I2 = 27%), so the fixed effects model was selected for this analysis. Results showed that the dyspnea score was not statistically significant after the intervention (MD = –0.76; 95% CI [–1.8, 0.27]; p = 0.15).
Anxiety: Three studies (Chen et al., 2015; Coats et al., 2013; Olivier et al., 2018) reported the effect of the intervention on anxiety. There was no heterogeneity in anxiety (I2 = 0%), so the fixed effects model was selected for this analysis (MD = –1.06; 95% CI [–2, –0.12]; p = 0.03). Results showed that home-based exercise training could improve anxiety in patients with lung cancer.
Depression: Three studies (Chen et al., 2015; Coats et al., 2013; Olivier et al., 2018) reported the effect of the intervention on depression. Analysis of these three studies demonstrated evidence of moderate heterogeneity (I2 = 49%), so the fixed effects model was selected for this analysis. Results showed that depression was not significantly different after the intervention (MD = –0.67; 95% CI [–1.71, 0.37]; p = 0.21).
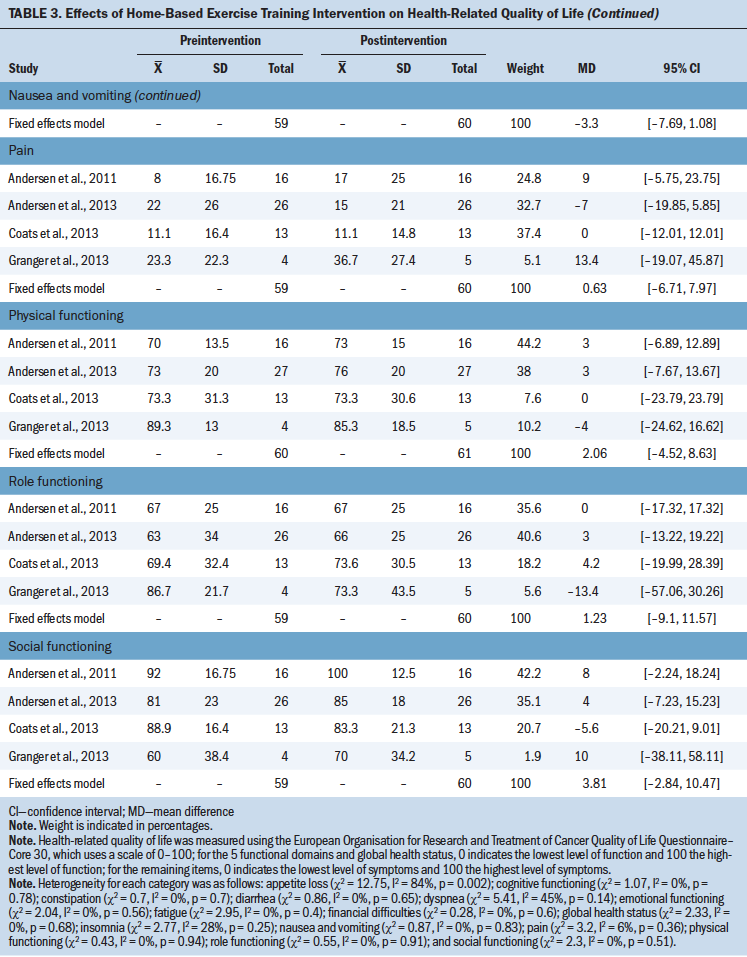
HRQOL: Five studies (Andersen et al., 2011, 2013; Arbane et al., 2011; Coats et al., 2013; Granger et al., 2013) reported the effect of the intervention on HRQOL, measured with the EORTC QLQ-C30. To analyze appetite loss, a random effects model was used on the basis of the high heterogeneity (I2 = 84%). Analysis for other domains employed a fixed effects model because the heterogeneity was less than 50%. Results showed that the intervention significantly improved emotional functioning (MD = 8.77; 95% CI [2.02, 15.51]; p = 0.01) and insomnia (MD = –13.33; 95% CI [–20.21, –6.44]; p = 0.0001). However, there were significant differences in the following domains:
• Global health status (MD = 1.21; 95% CI [–4.81, 7.22]; p = 0.69)
• Physical functioning (MD = 2.06; 95% CI [–4.52, 8.63]; p = 0.54)
• Role functioning (MD = 1.23; 95% CI [–9.1, 11.57]; p = 0.82)
• Cognitive functioning (MD = 4.76; 95% CI [–2.07, 11.58]; p = 0.17)
• Social functioning (MD = 3.81; 95% CI [–2.84, 10.47]; p = 0.26)
• Fatigue (MD = 3.66; 95% CI [–4.14, 11.46]; p = 0.36)
• Nausea and vomiting (MD = –3.3; 95% CI [–7.69, 1.08]; p = 0.14)
• Pain (MD = 0.63; 95% CI [–6.71, 7.97]; p = 0.87)
• Dyspnea (MD = –7.69; 95% CI [–16.14, 0.77]; p = 0.07)
• Appetite loss (MD = –17.22; 95% CI [–36.25, 1.8]; p = 0.08)
• Constipation (MD = 1.22; 95% CI [–4.13, 6.56]; p = 0.66)
• Diarrhea (MD = –4.7; 95% CI [–11.57, 2.17]; p = 0.18)
• Financial difficulties (MD = –1.51; 95% CI [–8.73, 5.71]; p = 0.68)
Sensitivity Analysis
To assess the stability of the meta-analysis, a sensitivity analysis was conducted. For dyspnea, the heterogeneity significantly decreased (I2 = 0%) when the Coats et al. (2013) study was removed, demonstrating that it was the main source of heterogeneity. In addition, the adjusted pooled estimates changed significantly (MD = –1.4; 95% CI [–2.81, 0.01]; p = 0.05). Similarly, the heterogeneity of depression significantly decreased (I2 = 0%) when the Olivier et al. (2018) study was removed. In addition, the adjusted pooled estimates changed significantly (MD = –1.33; 95% CI [–2.56, –0.1]; p = 0.03). Removal of the Arbane et al. (2011) study significantly decreased the heterogeneity of the appetite loss domain of the EORTC QLQ-C30 (I2 = 0%), demonstrating that it was the main source of heterogeneity. However, the adjusted pooled estimates did not experience a significant change (MD = –8; 95% CI [–19.14, 3.14]; p = 0.16).
Discussion
The results of the analysis indicate that home-based exercise training can improve exercise capacity and anxiety. However, it does not improve dyspnea, depression, or HRQOL. Ten studies (RCTs and non-RCTs) involving a total of 453 participants were included in the current systematic review. The home-based exercise training in the studies selected for inclusion involved aerobic training, resistance training, and breathing exercises. The frequency of exercise training ranged from once daily to once weekly.
Lung cancer is associated with impaired lung function and symptoms of lower exercise capacity. A prospective study by Kasymjanova et al. (2009) demonstrated that advanced stage lung cancer significantly reduced exercise capacity after chemotherapy. Exercise capacity is an independent predictor of survival in patients with lung cancer, with a 13% reduction in the risk of death when the 6MWD increases by 50 m (Jones et al., 2012). Exercise training may improve the skeletal muscle function of patients and effectively reduce oxidative stress in the body to increase exercise capacity (Mercken et al., 2005; Spruit et al., 2013). The current meta-analysis showed that home-based exercise training can significantly increase the 6MWD in patients with lung cancer. Similar to findings in the study by Claes, Buys, Budts, Smart, and Cornelissen (2017), exercise capacity increased with home-based exercise interventions.
Results of this meta-analysis demonstrate that home-based exercise training does not improve dyspnea. In contrast, the study by Bernardi, Pomidori, Cassutti, and Cogo (2018) showed that home-based exercise training could reduce the patient’s perception of dyspnea. The difference in results may be because the Bernadi et al. (2018) study used a metronome to maintain the walking speed, and the intervention effect was more significant. In the current meta-analysis, only three studies (Brocki et al., 2014; Coats et al., 2013; Hoffman et al., 2014) reported the outcome of dyspnea, and the sample size was smaller. In addition, in the study by Brocki et al. (2014), 43% of patients completed home-based exercise training. In the study by Coats et al. (2013), the recruitment rate was 50%, and the completion rate was 81%. Although all patients completed home-based exercise training in the study by Hoffman et al. (2014), it was a feasibility study involving only five patients. The small sample size and the different completion rates may affect the results of quantitative synthesis, and the current analysis of dyspnea should be interpreted with caution.
Following the sensitivity analysis on dyspnea, the current authors discovered that the study by Coats et al. (2013) was a source of heterogeneity. The home-based exercise training in the Coats et al. (2013) study was performed preoperatively and the length of the intervention was shorter (four weeks); both of these characteristics differ from the other two studies that reported the outcome of dyspnea (Brocki et al., 2014; Hoffman et al., 2014).
Patients with lung cancer generally have psychological problems, such as anxiety and depression, which may limit their ability to exercise and may impair their HRQOL (Chen et al., 2015; Liberati et al., 2009). The current meta-analysis employed HADS to assess anxiety and depression. Exercise training may reduce sympathetic nerve excitability and relieve anxiety and depression (Chen, Huang, Chien, & Cheng, 2017). The findings of the current meta-analysis show that home-based exercise training reduces anxiety. These results are similar to those from a study by Blacklock, Rhodes, Blanchard, and Gaul (2010) showing that exercise training could reduce anxiety.
However, the current authors did not observe a significant difference in depression. In the study by Chen et al. (2015), the participants received a walking exercise booklet, and 26 of 58 participants completed the intervention. In addition, three patients could not tolerate the moderate-intensity exercises during the first and second weeks, which has a negative impact on the fidelity of the intervention and possibly weakens the intervention effect. When the Olivier et al. (2018) study was omitted from the sensitivity analysis for depression, the results changed into a significant difference (p = 0.03). One of the potential explanations for this change is that participants in the Olivier et al. (2018) study had lung cancer and malignant pleural mesothelioma treated with chemotherapy, and only 47 of 243 eligible patients agreed to participate and finished the home-based exercise training. In addition, the approaches to follow-up may also be the cause of heterogeneity. Home visits were used for supervision in the Olivier et al. (2018) study, whereas telephone calls were used in the two other studies measuring depression (Chen et al., 2015; Coats et al., 2013). Additional high-quality RCTs are needed to verify the impact of home-based exercise training on depression.
Patients with lung cancer have a high symptom burden, with fatigue, pain, insomnia, and poor QOL (Mercadante et al., 2017; Morrison et al., 2017). Patients with lung cancer undergoing chemotherapy experience appetite loss, diarrhea, nausea, and vomiting (Ponticelli et al., 2017). These symptoms not only affect patients’ physical and mental health but also increase their financial burden. The current meta-analysis showed that home-based exercise training can improve the domains of emotional functioning and insomnia. Mercadante et al. (2017) reported that sleep quality is closely related to emotional functioning. Hartescu, Morgan, and Stevinson (2015) found that exercise improved insomnia and mood outcomes. Exercise may improve sleep through regulating proinflammatory cytokines, but other related mechanisms need additional research (Sprod et al., 2010).
Significant improvements were not observed in other domains of the EORTC QLQ-C30. The studies by Andersen et al. (2011, 2013) examined participants who for the most part were not eligible for surgery and who in general had more advanced disease and a worse prognosis. Arbane et al. (2011) reported only global health status, and Granger et al. (2013) did not report the domains of insomnia, appetite loss, constipation, diarrhea, and financial difficulties. These studies may affect the results of quantitative synthesis. Larger multicenter parallel RCTs are needed to verify the impact of home-based exercise training on HRQOL.
These studies have significant heterogeneity in the types of patients included (e.g., stage of cancer, treatment approaches), which requires caution in explaining the results. Because the included studies described lung cancer staging more broadly and the number of studies included was small, additional analysis was not conducted on patients with different stages of lung cancer. It is hoped that more studies will further investigate the impact of home-based exercise training on lung cancer at different stages.
Limitations
This systematic review and meta-analysis has several limitations. Because of the lack of RCTs on this subject, non-RCTs were included; these are more easily biased and may have affected the validity and reliability of the results. In addition, the sample size of 453 participants was generally smaller than that of other reviews, which could limit the analytical power. Differences in intervention methods, participants, and research designs inevitably led to clinical heterogeneity. Additional studies will need to be conducted to investigate this question. There also is no assessment of publication bias because of the small number of studies included. However, the novelty of the research field (the oldest article was published in 2011), with the addition of the differences in the results, suggests that publication bias did not likely influence results.
Implications for Nursing
Patients with lung cancer are at risk not only for symptom burden but also for comorbid conditions, which seriously impair the quality of life (Brunelli, Kim, Berger, & Addrizzo-Harris, 2013). The current meta-analysis showed that home-based exercise training may have beneficial effects on exercise capacity and anxiety. Nurses should examine the benefits of home-based exercise training and consider this intervention for patients. In the lung cancer setting, the effectiveness of the intervention appears to be highly influenced by the clinical features of participants (e.g., age, cancer type, cancer stage, treatment approaches) and follow-up methods.
When setting up a stronger and more effective nursing rehabilitation model, there are several factors that should be taken into account to maximize the results. The home-based interventions should be tailored toward the various physical states of individuals with lung cancer to maintain and improve function. For older patients with advanced lung cancer, a tolerable training method, such as walking, should be chosen. For patients with early-stage lung cancer and better physical status, a combination of multiple exercise methods, such as aerobic training, resistance training, and breathing exercises, should be chosen. In addition, nurses can send audiovisual materials about exercise training through the Internet; these materials should be designed to help patients with lung cancer learn more about exercises at home, so that they can maintain healthy behavior and promote rehabilitation. The approaches to follow-up in this review involved home visits, telephone calls, and logbooks. Future research may focus on follow-up methods using the Internet to observe the exercise training of the patient in a timely manner and to improve the effects of the intervention on patients (Inskip et al., 2018). 
Conclusion
This meta-analysis demonstrated that home-based exercise training may provide benefits for exercise capacity and anxiety. However, there were no significant improvements noted in dyspnea, depression, or HRQOL. Exercise is one of the key components of the current management of lung cancer, and patients should be encouraged to adhere to exercise training at home. Therefore, more high-quality RCTs on this topic should be included to further investigate the effects of home-based exercise training.
About the Author(s)
Ya-Qing Wang, MD, Xin Liu, MD, Ying-Ying Yin, MD, Rui-Chen Ma, MD, Zhuo Yang, MD, and Hui-Ping Cao, MD, are graduate students and Jiao Xie, PhD, is an associate professor, all in the School of Nursing at Jilin University in China. No financial relationships to disclose. All authors contributed to the conceptualization and design and to the manuscript preparation. Wang, Liu, Yin, Ma, and Xie completed the data collection and provided statistical support and analysis. Xie can be reached at 1761458270@qq.com, with copy to ONFEditor@ons.org. (Submitted December 2018. Accepted February 11, 2019.)
References
Andersen, A.H., Vinther, A., Poulsen, L.-L., & Mellemgaard, A. (2011). Do patients with lung cancer benefit from physical exercise? Acta Oncologica, 50, 307–313. https://doi.org/10.3109/0284186x.2010.529461
Andersen, A.H., Vinther, A., Poulsen, L.-L., & Mellemgaard, A. (2013). A modified exercise protocol may promote continuance of exercise after the intervention in lung cancer patients—A pragmatic uncontrolled trial. Supportive Care in Cancer, 21, 2247–2253. https://doi.org/10.1007/s00520-013-1781-z
Arbane, G., Tropman, D., Jackson, D., & Garrod, R. (2011). Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: Randomised controlled trial. Lung Cancer, 71, 229–234. https://doi.org/10.1016/j.lungcan.2010.04.025
Bernardi, E., Pomidori, L., Cassutti, F., & Cogo, A. (2018). Home-based, moderate-intensity exercise training using a metronome improves the breathing pattern and oxygen saturation during exercise in patients with COPD. Journal of Cardiopulminary Rehabilitation and Prevention, 38, E16–E18.
Blacklock, R., Rhodes, R., Blanchard, C., & Gaul, C. (2010). Effects of exercise intensity and self-efficacy on state anxiety with breast cancer survivors. Oncology Nursing Forum, 37, 206–212. https://doi.org/10.1188/10.onf.206-212
Borg, G.A. (1974). Perceived exertion. Exercise and Sport Sciences Reviews, 2, 131–153.
Brocki, B.C., Andreasen, J., Nielsen, L.R., Nekrasas, V., Gorst-Rasmussen, A., & Westerdahl, E. (2014). Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery—A randomized controlled trial. Lung Cancer, 83, 102–108. https://doi.org/10.1016/j.lungcan.2013.10.015
Brunelli, A., Charloux, A., Bolliger, C.T., Rocco, G., Sculier, J.-P., Varela, G., . . . Goldman, L. (2009). ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). European Respiratory Journal, 34, 17–41. https://doi.org/10.1183/09031936.00184308
Brunelli, A., Kim, A.W., Berger, K.I., & Addrizzo-Harris, D.J. (2013). Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest, 143(Suppl.),e166S–e190S. https://doi.org/10.1378/chest.12-2395
Chen, H.-M., Tsai, C.-M., Wu, Y.-C., Lin, K.-C., & Lin, C.-C. (2015). Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. British Journal of Cancer, 112, 438–445. https://doi.org/10.1038/bjc.2014.612
Chen, Y.-F., Huang, X.-Y., Chien, C.-H., & Cheng, J.-F. (2017). The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspectives in Psychiatric Care, 53, 329–336. https://doi.org/10.1111/ppc.12184
Claes, J., Buys, R., Budts, W., Smart, N., & Cornelissen, V.A. (2017). Longer-term effects of home-based exercise interventions on exercise capacity and physical activity in coronary artery disease patients: A systematic review and meta-analysis. European Journal of Preventive Cardiology, 24, 244–256. https://doi.org/10.1177/2047487316675823
Cleeland, C.S., Zhao, F., Chang, V.T., Sloan, J.A., O’Mara, A.M., Gilman, P.B., . . . Fisch, M.J. (2013). The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer, 119, 4333–4340. https://doi.org/10.1002/cncr.28376
Coats, V., Maltais, F., Simard, S., Fréchette, É., Tremblay, L., Ribeiro, F., & Saey, D. (2013). Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Canadian Respiratory Journal, 20, e10–e16. https://doi.org/10.1155/2013/291059
Duijts, S.F.A., van Beurden, M., Oldenburg, H.S.A., Hunter, M.S., Kieffer, J.M., Stuvier, M.M., . . . Aaronson, N.K. (2012). Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: Results of a randomized, controlled, multicenter trial. Journal of Clinical Oncology, 30, 4124–4133. https://doi.org/10.1200/JCO.2012.41.8525
Giese-Davis, J., Collie, K., Rancourt, K.M.S., Neri, E., Kraemer, H.C., & Spiegel, D. (2011). Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. Journal of Clinical Oncology, 29, 413–420. https://doi.org/10.1200/jco.2010.28.4455
Goldstraw, P., Chansky, K., Crowley, J., Rami-Porta, R., Asamura, H., Eberhardt, W.E.E., . . . Bolejack, V. (2016). The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. Journal of Thoracic Oncology, 11, 39–51. https://doi.org/10.1016/j.jtho.2015.09.009
Granger, C.L., Chao, C., McDonald, C.F., Berney, S., & Denehy, L. (2013). Safety and feasibility of an exercise intervention for patients following lung resection: A pilot randomized controlled trial. Integrative Cancer Therapies, 12, 213–224. https://doi.org/10.1177/1534735412450461
Granger, C.L., McDonald, C.F., Berney, S., Chao, C., & Denehy, L. (2011). Exercise intervention to improve exercise capacity and health related quality of life for patients with non-small cell lung cancer: A systematic review. Lung Cancer, 72, 139–153. https://doi.org/10.1016/j.lungcan.2011.01.006
Hamilton, D.M., & Haennel, R.G. (2000). Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. Journal of Cardiopulmonary Rehabilitation, 20, 156–164. https://doi.org/10.1097/00008483-200005000-00003
Hartescu, I., Morgan, K., & Stevinson, C.D. (2015). Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: A randomized controlled trial. Journal of Sleep Research, 24, 526–534. https://doi.org/10.1111/jsr.12297
Higgins, J.P.T., & Green, S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions [v.5.1.0]. Retrieved from http://handbook-5-1.cochrane.org
Hoffman, A.J., Brintnall, R.A., von Eye, A., Jones, L.W., Alderink, G., Patzelt, L.H., & Brown, J.K. (2014). A rehabilitation program for lung cancer patients during postthoracotomy chemotherapy. OncoTargets and Therapy, 2014, 415–423. https://doi.org/10.2147/ott.s57262
Hong, Q.-Y., Wu, G.-M., Qian, G.-S., Hu, C.-P., Zhou, J.-Y., Chen, L.-A., . . . Bai, C.-X. (2015). Prevention and management of lung cancer in China. Cancer, 121(Suppl. 17), 3080–3088. https://doi.org/10.1002/cncr.29584
Inskip, J.A., Novak Lauscher, H., Li, L.C., Dumont, G.A., Garde, A., Ho, K., . . . Camp, P.G. (2018). Patient and health care professional perspectives on using telehealth to deliver pulmonary rehabilitation. Chronic Respiratory Disease, 15, 71–80. https://doi.org/10.1177/1479972317709643
Jones, L.W., Hornsby, W.E., Goetzinger, A., Forbes, L.M., Sherrard, E.L., Quist, M., . . . Abernethy, A.P. (2012). Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer, 76, 248–252. https://doi.org/10.1016/j.lungcan.2011.10.009
Jung, J.Y., Lee, J.M., Kim, M.S., Shim, Y.M., Zo, J.I., & Yun, Y.H. (2018). Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psycho-Oncology, 27, 465–470. https://doi.org/10.1002/pon.4513
Kasymjanova, G., Correa, J.A., Kreisman, H., Dajczman, E., Pepe, C., Dobson, S., . . . Small, D. (2009). Prognostic value of the six-minute walk in advanced non-small cell lung cancer. Journal of Thoracic Oncology, 4, 602–607. https://doi.org/10.1097/JTO.0b013e31819e77e8
Lai, Y., Su, J., Qiu, P., Wang, M., Zhou, K., Tang, Y., & Che, G. (2017). Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: A randomized trial. Interactive Cardiovascular and Thoracic Surgery, 25, 476–483. https://doi.org/10.1093/icvts/ivx141
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P.A., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Lou, V.W.Q., Chen, E.J., Jian, H., Zhou, Z., Zhu, J., Li, G., & He, Y. (2017). Respiratory symptoms, sleep, and quality of life in patients with advanced lung cancer. Journal of Pain and Symptom Management, 53, 250–256.e1. https://doi.org/10.1016/j.jpainsymman.2016.09.006
Mercadante, S., Adile, C., Ferrera, P., Masedu, F., Valenti, M., & Aielli, F. (2017). Sleep disturbances in advanced cancer patients admitted to a supportive/palliative care unit. Supportive Care in Cancer, 25, 1301–1306. https://doi.org/10.1007/s00520-016-3524-4
Mercken, E.M., Hageman, G.J., Schols, A.M.W.J., Akkermans, M.A., Bast, A., & Wouters, E.F.M. (2005). Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 172, 994–1001. https://doi.org/10.1164/rccm.200411-1580OC
Morrison, E.J., Novotny, P.J., Sloan, J.A., Yang, P., Patten, C.A., Ruddy, K.J., & Clark, M.M. (2017). Emotional problems, quality of life, and symptom burden in patients with lung cancer. Clinical Lung Cancer, 18, 497–503. https://doi.org/10.1016/j.cllc.2017.02.008
Mujovic, N., Mujovic, N., Subotic, D., Marinkovic, M., Milovanovic, A., Stojsic, J., . . . Nikolic, D. (2014). Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Archives of Medical Science, 10, 68–75. https://doi.org/10.5114/aoms.2013.32806
Nici, L., Donner, C., Wouters, E., Zuwallack, R., Ambrosino, N., Bourbeau, J., . . . Troosters, T. (2006). American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine, 173, 1390–1413. https://doi.org/10.1164/rccm.200508-1211ST
Olivier, C., Grosbois, J.-M., Cortot, A.B., Peres, S., Heron, C., Delourme, J., . . . Le Rouzic, O. (2018). Real-life feasibility of home-based pulmonary rehabilitation in chemotherapy-treated patients with thoracic cancers: A pilot study. BMC Cancer, 18, 178. https://doi.org/10.1186/s12885-018-4102-6
O’Shea, S.D., Taylor, N.F., & Paratz, J.D. (2009). Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: A systematic review. Chest, 136, 1269–1283. https://doi.org/10.1378/chest.09-0029
Ponticelli, E., Clari, M., Frigerio, S., De Clemente, A., Bergese, I., Scavino, E., . . . Sacerdote, C. (2017). Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. European Journal of Cancer Care, 26, e12633. https://doi.org/10.1111/ecc.12633
Quist, M., Rørth, M., Langer, S., Jones, L.W., Laursen, J.H., Pappot, H., . . . Adamsen, L. (2012). Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: A pilot study. Lung Cancer, 75, 203–208. https://doi.org/10.1016/j.lungcan.2011.07.006
Siegel, R.L., Miller, K.D., & Jemal, A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68, 7–30. https://doi.org/10.3322/caac.21442
Slim, K., Nini, E., Forestier, D., Kwiatkowski, F., Panis, Y., & Chipponi, J. (2003). Methodological index for non-randomizedstudies (MINORS): Development and validation of a new instrument. Anz Journal of Surgery, 73, 712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Sprod, L.K., Palesh, O.G., Janelsins, M.C., Peppone, L.J., Heckler, C.E., Adams, M.J., . . . Mustian, K.M. (2010). Exercise, sleep quality, and mediators of sleep in breast and prostate cancer patients receiving radiation therapy. Community Oncology, 7, 463–471.
Spruit, M.A., Singh, S.J., Garvey, C., ZuWallack, R., Nici, L., Rochester, C., . . . Wouters, E.F.M. (2013). An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine, 188, e13–e64. https://doi.org/10.1164/rccm.201309-1634ST
Temel, J.S., Greer, J.A., Goldberg, S., Vogel, P.D., Sullivan, M., Pirl, W.F., . . . Smith, M.R. (2009). A structured exercise program for patients with advanced non-small cell lung cancer. Journal of Thoracic Oncology, 4, 595–601. https://doi.org/10.1097/JTO.0b013e31819d18e5
Yang, M., Zhong, J.-D., Zhang, J.-E., Huang, X.-X., Li, C.-Z., Hong, Z.-X., & Zhang, S.-W. (2018). Effect of the self-efficacy-enhancing active cycle of breathing technique on lung cancer patients with lung resection: A quasi-experimental trial. European Journal of Oncology Nursing, 34, 1–7. https://doi.org/10.1016/j.ejon.2018.02.009
Zigmond, A.S., & Snaith, R.P. (1983). The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica, 67, 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x




