Childhood Cancer Symptom Cluster: Leukemia and Health-Related Quality of Life
Objectives: To examine the relationship of the Childhood Cancer Symptom Cluster–Leukemia (CCSC-L) with health-related quality of life (HRQOL).
Sample & Setting: 327 children receiving treatment for acute lymphoblastic leukemia from four pediatric oncology programs across the United States.
Methods & Variables: Participants completed fatigue, sleep disturbance, pain, nausea, and depression symptom questionnaires at four time points; these symptoms comprised the CCSC-L. HRQOL was measured at the start of postinduction therapy and then at the start of maintenance therapy. Relationships between the CCSC-L and HRQOL scores were examined with longitudinal parallel-process modeling.
Results: The mean HRQOL significantly increased over time (p < 0.001). The CCSC-L had a significant negative association with HRQOL scores at the start of postinduction therapy (beta = –0.53, p < 0.005) and the start of maintenance therapy (beta = –0.33, p < 0.015). Participants with more severe symptoms in the CCSC-L over time had significantly lower HRQOL at the start of maintenance therapy (beta = –0.42, p < 0.005).
Implications for Nursing: Nurses are pivotal in providing management strategies to minimize symptom severity that may improve HRQOL.
Jump to a section
About 80% of children with cancer endure at least one symptom during treatment; more commonly, these children experience multiple symptoms throughout treatment (Buckner et al., 2014; Hockenberry et al., 2017). Symptoms of fatigue, nausea, pain, sleep disturbances, and depression commonly occur among children undergoing cancer treatment (Daniel, Li, Kloss, Reilly, & Barakat, 2016; Kestler & LoBiondo-Wood, 2012; Rodgers, Hooke, Ward, & Linder, 2016). A study by Hockenberry et al. (2017) evaluating the trajectory of symptoms among children with acute lymphoblastic leukemia (ALL) confirmed that sleep disturbance and nausea persisted during postinduction chemotherapy treatment, and fatigue, pain, and depression decreased but never completely resolved during this time. ALL is the most common type of childhood cancer and requires about three years of chemotherapy treatment (Scheurer, Lupo, & Bondy, 2016). ALL treatment is divided into three phases: induction therapy that starts urgently after the cancer diagnosis and lasts about one month; postinduction therapy (also referred to as consolidation or intensification therapy) that begins after induction therapy and includes at least eight months of intensified treatment; and maintenance therapy that starts after postinduction therapy and consists of less intensive treatment for about two years (Margolin, Rabin, Steuber, & Poplack, 2016). During postinduction therapy, children receive intensive treatment with several courses of chemotherapy that are associated with numerous symptoms (Hockenberry et al., 2014; Margolin et al., 2016). Children describe symptoms as the worst part of cancer treatment, noting that symptoms cause distress and increase suffering (Ameringer, Elswick, Shockey, & Dillon, 2013; Woodgate, 2008).
Children with ALL have low health-related quality of life (HRQOL) during their cancer treatment (Mitchell et al., 2016; Sung et al., 2011; van Litsenburg et al., 2014). Despite the prevalence of symptoms and low HRQOL, only a few studies have evaluated the relationship between symptoms and HRQOL in children undergoing treatment for ALL. Three cross-sectional studies revealed an association of a single symptom, fatigue, with poor HRQOL in children who were receiving treatment for any type of cancer (Al-Gamal & Long, 2016; Nunes et al., 2017; Pan, Wu, & Wen, 2017). Another cross-sectional study of 61 children receiving myelosuppressive chemotherapy found poor HRQOL scores among children with higher symptom distress scores (Baggott et al., 2011). Finally, lower HRQOL scores were noted among children with high symptoms related to their oral mucositis compared to children with low oral mucositis symptoms (Cheng, Lee, Li, Yuen, & Epstein, 2012). These cross-sectional studies provide a snapshot of the relationship between symptoms and HRQOL; however, symptoms are dynamic, and descriptions of the relationship of multiple symptoms and HRQOL are missing from the literature.
Symptoms rarely occur alone, and there is an increased priority to identify co-occurring symptoms in children with cancer, referred to as symptom clusters. Baggott, Cooper, Marina, Matthay, and Miaskowski (2012) identified three clusters (chemotherapy sequelae cluster, mood disturbance cluster, and neuropsychological discomfort cluster) among 131 children receiving cancer therapy. Likewise, Atay, Conk, and Bahar (2012) noted distinct symptom clusters among 54 adolescents at one, two, and three months postdiagnosis. Hockenberry, Hooke, McCarthy, and Gregurich (2011) noted two clusters—(a) fatigue and depression and (b) nausea, sleep disturbance, and performance status—among 67 children receiving cisplatin, doxorubicin, or ifosfamide for their cancer treatment.
Buckner et al. (2014) were among the first to use an advanced modeling technique, latent profile analysis, to characterize similar levels of symptom severity with functional outcomes in 200 children who were receiving or had completed therapy for a variety of cancer diagnoses. The authors identified that children with high symptom severity, including anxiety, depression, fatigue, and pain, reported the lowest functional outcomes, including peer relationships, upper extremity physical functioning, and mobility. Although HRQOL likely is associated with symptom clusters, no study has evaluated these relationships in children receiving leukemia therapy.
The current authors identified a symptom cluster of fatigue, sleep disturbance, pain, nausea, and depression, referred to as the Childhood Cancer Symptom Cluster–Leukemia (CCSC-L), among 327 children receiving treatment for ALL (Hooke et al., 2018). In addition, the CCSC-L was noted to act as a mediator between physical activity and cognition/memory (Hooke et al., 2018). The purpose of this article is to extend the analysis by examining the relationship between CCSC-L and HRQOL.
Methods
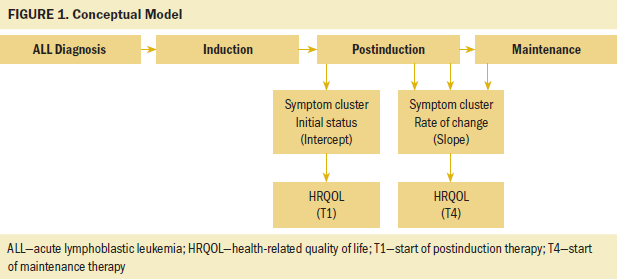
A repeated-measures design was used for this prospective study. This work was part of a larger study funded by the National Institutes of Health to characterize and explore associations of the phenotypic and genotypic characteristics in children experiencing symptoms related to leukemia treatment. The focus of this analysis is to identify the longitudinal association of the CCSC-L and HRQOL among children receiving ALL therapy. Participants completed symptom questionnaires at four time points during their postinduction treatment (T1 is start of postinduction therapy, T2 is four months postinduction therapy, T3 is eight months postinduction therapy, and T4 is start of maintenance therapy). HRQOL was measured at the start of postinduction therapy, then again at the start of maintenance therapy; the time interval between these two measures was about 12 months but varied depending on individual treatment delays. The conceptual model was developed by the authors and is illustrated in Figure 1. The figure represents data collected during ALL treatment and the potential relationship of HRQOL with the initial level of symptoms (cluster intercept) and the rate of change in symptoms over time (cluster slope).
Setting and Sample
Participants were recruited from four pediatric oncology programs across the United States, including one site in the Southwest, one in the northern section of the Midwest, one in the southern section of the Midwest, and one on the East Coast. Potential participants were invited to participate in the study if they were aged 3–18 years, were receiving first-time treatment according to an ALL protocol, and were fluent in English or Spanish. Exclusion criteria consisted of patients with relapsed ALL or any cognitive disability that was established before the cancer diagnosis.
All participants were treated on a lymphoblastic leukemia protocol and received similar therapy. Postinduction therapy involved several courses of treatment that included asparaginase, methotrexate, vincristine, doxorubicin, corticosteroid, cytarabine, and mercaptopurine. Intrathecal methotrexate was given on day 1 of each 12-week cycle.
Measures
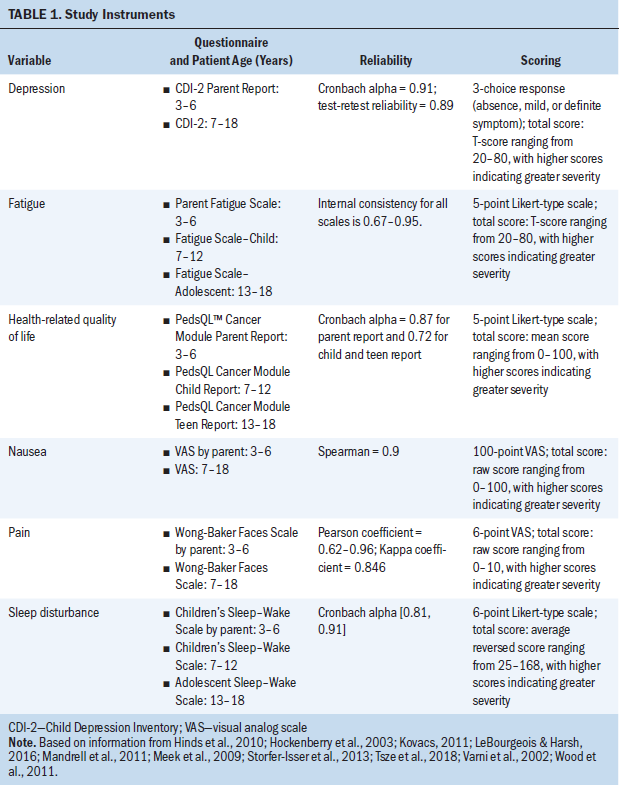
Severity of each of the five symptoms within the CCSC-L was measured individually with self-reported questionnaires for children aged 7 years or older, or parent-proxy questionnaires for children aged from 3 to 6 years. Each questionnaire is listed in Table 1, along with the established reliability and validity. To be consistent with the scoring direction of other symptom measures, scores on the Sleep–Wake Scale were reversed and the scale was renamed “sleep disturbance” so that higher scores for each symptom represented higher severity of the symptom.
In the current authors’ previous work, exploratory factor analysis demonstrated significant relationships among all the symptoms in the cluster (factor loadings from 0.37–0.91) throughout postinduction therapy and the start of maintenance therapy, establishing the CCSC-L (Hockenberry et al., 2017). The factor score of the CCSC-L was a composite score (or weighted average) of the values in the five symptom measures (i.e., fatigue, sleep disturbance, pain, nausea, and depression). The weights were determined by the one-factor solution in the exploratory factor analysis of the five symptom measures (Hockenberry et al., 2017). The factor score of the CCSC-L is a continuous variable, and the higher the factor score is, the more severe the CCSC-L.
HRQOL was measured with a self-reported questionnaire or parent-proxy questionnaire. Higher scores signified better HRQOL.
Procedure
Approval was obtained from the institutional review board at each site: Duke University in Durham, North Carolina; Texas Children’s Hospital/Baylor College of Medicine in Houston; University of Arizona in Tucson; and Children’s Minnesota in Minneapolis. Eligible patients and their parents were introduced to the study, initially by a provider known to them and then by a member of the study team. If the patient and parent agreed to participate, parents of patients aged younger than 18 years provided written consent, patients aged from 7 to 11 years provided verbal assent, and patients aged from 12 to 17 years provided written assent. Patients aged 18 years were consented.
All four time points for data collection occurred during a routine cancer center clinic visit or while hospitalized. At each time point, parents and patients were asked if they were willing to continue with the study and completed the questionnaires if agreed. Parent or patients who were unwilling to complete the questionnaires were asked if data could be collected at another time, and a future date was determined. All questionnaires were administered electronically in English or Spanish on a tablet computer (i.e., iPad). Demographic and treatment information was obtained from the medical record.
Data Analysis
Descriptive statistics were used to summarize the sample characteristics, symptom scores, and HRQOL scores over time. The initial sample consisted of 329 participants; however, 2 participants had missing data on all symptom measures and were excluded from the analysis. The remaining 327 participants had no missing data on demographic variables but some intermittent missing data across the four time points on CCSC-L and HRQOL scores. The missing pattern was verified by the Little’s (1988) test as missing completely at random, and no further missing data treatment was necessary. The intermittent missing data were automatically dealt with by the multilevel modeling technique in the two-step longitudinal parallel-process (LPP).
Relationships between the CCSC-L and HRQOL scores were examined using LPP (Cheong, MacKinnon, & Khoo, 2003). LPP is a two-step modeling technique on longitudinal data. In the first step, the growth parameters of each longitudinal variable (the intercept [or initial status] and the slope [or rate of change]) are estimated from multilevel modeling. In this step, intermittent missing data across time can be easily handled by the expectation-maximization algorithm in the multilevel modeling technique, and, if necessary, covariates can be controlled in the multilevel modeling. In this study, sociodemographic variables (e.g., age, sex, race/ethnicity) and leukemia risk levels were controlled when estimating the growth parameters. In the second step of LPP, structural equation modeling (SEM) (Kline, 2010) was used for examining the longitudinal relationships among the variables. The longitudinal relationships are captured by the causal paths among the growth parameters of each longitudinal variable (e.g., the intercept and the slope).
Guided by the literature on SEM (Hu & Bentler, 1999; Kline, 2010), the model-fit indices used for testing the model fit for the SEM were chi-square of the estimated model (chi-square), goodness of fit index (GFI), normed fit index (NFI), incremental fit index (IFI), relative fit index (RFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA). A nonsignificant chi-square value (e.g., p > 0.05) suggests a good overall model fit to the data, whereas RMSEA should be less than 0.06. For GFI, NFI, IFI, RFI, and CFI, values higher than 0.9 indicate a good fit to the data.
Results
Characteristics of the 327 participants have been previously described (Hooke et al., 2018) but primarily consisted of non-Hispanic ethnicity (n = 172, 53%) and male gender (n = 170, 52%). About 45% (n = 148) of participants were aged 3–6 years, 34% (n = 110) were aged 7–12 years, and 21% (n = 69) were aged 13–18 years. These sample characteristics are similar to children diagnosed with ALL throughout the United States; ALL is more common in those with Hispanic ethnicity, has a slight male predominance, and is less common among adolescents (Rabin, Gramatges, Margolin, & Poplack, 2016).
HRQOL
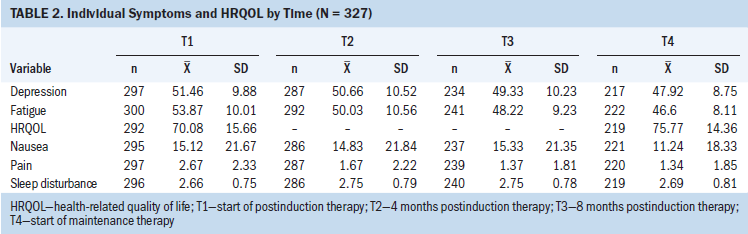
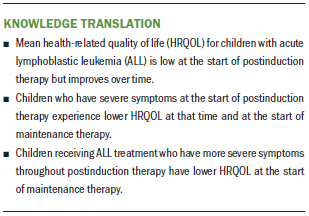
Overall, the mean HRQOL significantly increased from the start of postinduction therapy (T1) to the start of maintenance therapy (T4). The mean HRQOL at T1 was 70.08 (SD = 15.66), and the mean HRQOL at T4 was 75.77 (SD = 14.36) (p < 0.001) (see Table 2), with a close to medium effect (Cohen’s d = 0.4). This relationship is also demonstrated by the positive, significant path coefficient of HRQOL from T1 to T4 noted in Figure 2 (beta = 0.22, p < 0.008).
Relationships Between CCSC-L and HRQOL
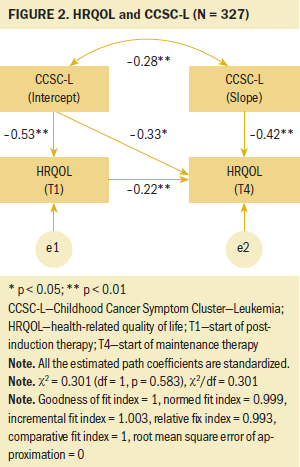
The results from the SEM demonstrated a good model fit to the data with satisfactory model-fit indices (chi-square[1] = 0.3, p = 0.583; GFI = 1, NFI = 1, IFI = 0.99, RFI = 0.96, CFI = 1, RMSEA < 0.001). For participants who had more severe symptoms in the CCSC-L at the start of postinduction therapy (intercept), the severity of their symptoms within the CCSC-L reduced faster (slope) than those who had less severe symptoms in the CCSC-L at the start of postinduction therapy (r = –0.28, p < 0.007). As for the relationships between the symptom cluster and HRQOL, a significant negative association was noted for HRQOL scores with the intercept of the CCSC-L at the start of postinduction therapy (T1) (beta = –0.53, p < 0.005) and the start of maintenance therapy (T4) (beta = –0.33, p < 0.015). Participants with more severe symptoms in the CCSC-L at the start of postinduction therapy experienced lower HRQOL at the start of postinduction therapy and the start of maintenance therapy. Similarly, the slope of the CCSC-L was negatively associated with HRQOL scores at the start of maintenance therapy (T4) (beta = –0.42, p < 0.005). Participants with more severe symptoms in the CCSC-L over time had significantly lower HRQOL at the start of postinduction therapy; this rate of change over time in the symptom cluster is represented in the slope of the CCSC-L.
Discussion
This study demonstrated a statistically significant improvement in HRQOL among children with ALL during 12 months of treatment. Improvement in HRQOL is similar to other studies that reported a low HRQOL among children with ALL during induction therapy that slowly improved during subsequent phases of therapy (Eiser et al., 2017; Furlong et al., 2012; Mitchell et al., 2016). This improvement would be expected as leukemia chemotherapy treatment becomes less intensive over the first 12–14 months of treatment after the more intensive early phases of induction and consolidation. In the current study, the 5.69 change in the PedsQL™ HRQOL score was clinically significant because it was greater than the published change threshold of 4.5 needed for a minimal clinically meaningful difference (Varni, Limbers, & Burwinkle, 2007). Despite the improvement, the improved mean score of 75.77 was still lower than a healthy sample of children who report HRQOL with a mean score of 83 on a generic scale (Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002). Overall, children receiving treatment for ALL have a poor HRQOL during postinduction therapy through the start of maintenance therapy. These children require ongoing assessment to identify factors negatively influencing their quality of life.
This study identified a significant longitudinal relationship between the CCSC-L and HRQOL. The LPP model was instrumental in evaluating the longitudinal relationships between two processes over time (i.e., CCSC-L and HRQOL) because it allowed an evaluation of the relationship between rates of change in the two variables, which can be measured at different time points (Cheong et al., 2003; Sousa, Kwok, Schmiege, & West, 2014). This analysis revealed that children who experienced more severe symptoms within the CCSC-L at baseline had lower HRQOL scores at the start of postinduction therapy and the start of maintenance therapy. Likewise, children with more severe symptoms within the CCSC-L over time had lower HRQOL at the start of maintenance therapy. Sleep disturbances occurring for more than one month have been associated with worse HRQOL in children receiving treatment for ALL (Daniel et al., 2016), but symptoms rarely occur in isolation (Aktas, 2013). Of importance from these findings is the fact that a symptom cluster, not just a single symptom, was negatively associated with HRQOL. Awareness of and response to symptom clusters allows healthcare providers to provide comprehensive interventions to minimize or alleviate the factors negatively affecting patients.
Findings from this study are significant because the variable influencing HRQOL in this study can be managed with appropriate intervention strategies. Symptom severity within the CCSC-L can be minimized through symptom management interventions, such as medication or psychoeducation (Lopes-Junior et al., 2016; Nunes et al., 2018). Other factors known to negatively influence HRQOL among children with ALL include older age (Eiser et al., 2017; van Litsenburg et al., 2014) and corticosteroid therapy (Daniel et al., 2016; Fardell et al., 2017) that cannot be altered. Therefore, clinical and research efforts should focus on relieving the modifiable variable (i.e., the symptom cluster) by implementing strategies early in treatment that will minimize or ameliorate the symptom cluster, thereby improving the HRQOL.
Limitations
A limitation of this study includes a sample consisting of a wide range of ages. Studies have found negative associations with older age and HRQOL (Eiser et al., 2017; van Litsenburg et al., 2014); however, additional studies are needed to identify symptom clusters among specific age groups (Rodgers et al., 2016). The researchers also recognize that other variables may influence HRQOL and symptom severity as children begin the maintenance phase of leukemia treatment, and these merit investigation in the future. Potential variables include adaptation to the diagnosis of cancer, improved ability and confidence in managing symptoms, and relief at maintaining remission and entering less demanding cycles of treatment. In addition, the sample in this study excluded children with cognitive disabilities. Although this study was designed to obtain self-reported symptom data among the participants, future studies could obtain objective and proxy symptom data among children with cognitive disabilities to evaluate symptom clusters and HRQOL. Ultimately, future studies should evaluate the relationship of symptom clusters and HRQOL during cancer therapy among specific groups of children with defined characteristics (i.e. age, preexisting conditions).
A strength of this study is the evaluation of symptom clusters and HRQOL during a specific phase of cancer therapy; however, future studies should be conducted in children with ALL beyond the start of maintenance therapy to evaluate the duration of the relationship of CCSC-L and HRQOL. Evaluation of symptom clusters and HRQOL in children during all phases of treatment can identify if changes occur over time, which can assist healthcare providers who are providing symptom management.
The differences in adherence to symptom management strategies may have affected the symptom cluster and HRQOL in this study. Although supportive care for symptoms is similar for patients during ALL therapy, the uptake of a symptom management plan is highly individualized among patients. For example, the same symptom treatment may have been provided to two patients in this study with nausea, but one patient may have been adherent to the regimen while the other patient could have ignored the plan. The potential influence of symptom management strategies should be considered in future studies.
Implications for Nursing
Symptom assessment and management is integral to nursing care. Nurses are often familiar with the incidence of individual symptoms but may not be aware of the interaction of multiple symptoms or how symptom cluster is affecting a child’s life. Questions during the assessment about the interaction of multiple symptoms can provide a more comprehensive understanding of the symptom experience. For example, if a child with ALL is reporting nausea and pain during postinduction therapy, the nurse should ask about fatigue, sleep disturbances, and depression, because these symptoms commonly cluster together. In addition, an understanding of how the symptom cluster is affecting the child’s lifestyle will identify important areas for symptom management. For example, rather than asking the child to rate the severity of a specific symptom, questions such as, “What is bothering you most during the day (or night)?” or “What is keeping you from doing what you want?” can provide an opportunity for the child to discuss multiple symptoms and start a dialogue to help distinguish the most distressing symptoms. With this information, nurses can advocate for symptom management strategies focused on the most bothersome symptoms for the child. Customizing symptom management strategies may increase the child’s HRQOL more than the typical improvement.
Conclusion
Children receiving treatment for ALL have a low HRQOL, but healthcare providers can focus efforts on management strategies that can improve HRQOL. The CCSC-L was negatively associated with HRQOL during the postinduction treatment. To improve HRQOL, symptom management strategies should focus on reducing or alleviating fatigue, sleep disturbances, pain, nausea, and depression. Future research should focus on the effectiveness of the symptom management strategies and identify other modifiable factors that significantly affect HRQOL.
The authors dedicate this work to the memory of Cheryl C. Rodgers, PhD, RN, CPNP, CPON®, who was the lead author for this manuscript and led the team in its development. Rodgers died unexpectedly during the publication of this work.
About the Author(s)
Cheryl C. Rodgers, PhD, RN, CPNP, CPON®, was an associate professor in the School of Nursing at Duke University in Durham, NC; Mary C. Hooke, PhD, APRN, PCNS, CPON®, FAAN, is an associate professor in the School of Nursing at the University of Minnesota in Minneapolis; Olga A. Taylor, MPH, is a clinical research manager in the College of Medicine at Baylor University in Houston, TX; Kari M. Koerner, MPH, CHES, is a senior research specialist in the College of Nursing at the University of Arizona in Tucson; Pauline A. Mitby, MPH, is a clinical research manager in the Pediatric Cancer and Blood Disorders Program at Children’s Minnesota in Minneapolis; Ida M. Moore, PhD, RN, FAAN, is an Ann Furrow professor of nursing in the College of Nursing and director of the Biobehavioral Health Sciences Division at the University of Arizona; Michael E. Scheurer, PhD, MPH, is an associate professor in the College of Medicine at Baylor University; and Marilyn J. Hockenberry, PhD, RN, FAAN, is a Bessie Baker professor emeritus of nursing and Wei Pan, PhD, is an associate professor, both in the School of Nursing at Duke University. This research was funded, in part, by a grant (RO1CA1693398) from the National Institutes of Health and through support from the Alex’s Lemonade Stand Foundation. Moore, Scheurer, and Hockenberry contributed to the conceptualization and design. Hooke, Taylor, Koerner, Mitby, Moore, and Scheurer completed the data collection. Moore and Pan provided statistical support. Rodgers, Hooke, Moore, Hockenberry, and Pan provided the analysis. All authors contributed to the manuscript preparation. Hooke can be reached at hook0035@umn.edu, with copy to ONFEditor@ons.org. (Submitted May 2018. Accepted October 9, 2018.)
References
Aktas, A. (2013). Cancer symptom clusters: Current concepts and controversies. Current Opinion in Supportive and Palliative Care, 7, 38–44. https://doi.org/10.1097/SPC.0b013e32835def5b
Al-Gamal, E., & Long, T. (2016). Health-related quality of life and its association with self-esteem and fatigue among children diagnosed with cancer. Journal of Clinical Nursing, 25, 3391–3399. https://doi.org/10.1111/jocn.13467
Ameringer, S., Elswick, R.K., Jr., Shockey, D.P., & Dillon, R. (2013). A pilot exploration of symptom trajectories in adolescents with cancer during chemotherapy. Cancer Nursing, 36, 60–71. https://doi.org/10.1097/NCC.0b013e318250da1a
Atay, S., Conk, Z., & Bahar, Z. (2012). Identifying symptom clusters in paediatric cancer patients using the Memorial Symptom Assessment Scale. European Journal of Cancer Care, 21, 460–468. https://doi.org/10.1111/j.1365-2354.2012.01324.x
Baggott, C., Cooper, B.A., Marina, N., Matthay, K.K., & Miaskowski, C. (2012). Symptom cluster analyses based on symptom occurrence and severity ratings among pediatric oncology patients during myelosuppressive chemotherapy. Cancer Nursing, 35, 19–28. https://doi.org/10.1097/NCC.0b013e31822909fd
Baggott, C.R., Dodd, M., Kennedy, C., Marina, N., Matthay, K.K., Cooper, B., & Miaskowski, C. (2011). An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Supportive Care in Cancer, 19, 353–361. https://doi.org/10.1007/s00520-010-0824-y
Buckner, T.W., Wang, J., DeWalt, D.A., Jacobs, S. Reeve, B.B. & Hinds, P.S. (2014). Patterns of symptoms and functional impairments in children with cancer. Pediatric Blood and Cancer, 61, 1282–1288. https://doi.org/10.1002/pbc.25029
Cheng, K.K., Lee, V., Li, C.H., Yuen, H.L. & Epstein, J.B. (2012). Oral mucositis in pediatric and adolescent patients undergoing chemotherapy: The impact of symptoms on quality of life. Supportive Care in Cancer, 20, 2335–2342. https://doi.org/10.1007/s00520-011-1343-1
Cheong, J., MacKinnon, D.P., & Khoo, S.T. (2003). Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling, 10, 238–262. https://doi.org/10.1207/S15328007SEM1002_5
Daniel, L.C., Li, Y., Kloss, J.D., Reilly, A.F., & Barakat, L.P. (2016). The impact of dexamethasone and prednisone on sleep in children with acute lymphoblastic leukemia. Supportive Care in Cancer, 24, 3897–3906. https://doi.org/10.1007/s00520-016-3234-y
Eiser, C., Stride, C.B., Vora, A., Goulden, N., Mitchell, C., Buck, G., . . . Jenney, M.E.M. (2017). Prospective evaluation of quality of life in children treated in UKALL 2003 for acute lymphoblastic leukaemia: A cohort study. Pediatric Blood and Cancer, 64. https://doi.org/10.1002/pbc.26615
Fardell, J.E., Vetsch, J., Trahair, T., Mateos, M.K., Grootenhuis, M.A., Touyz, L.M., . . . Wakefield, C.E. (2017). Health-related quality of life of children on treatment for acute lymphoblastic leukemia: A systematic review. Pediatric Blood and Cancer, 64. https://doi.org/10.1002/pbc.26489
Furlong, W., Rae, C., Feeny, D., Gelber, R.D., Laverdiere, C., Michon, B., . . . Barr, R. (2012). Health-related quality of life among children with acute lymphoblastic leukemia. Pediatric Blood and Cancer, 59, 717–724. https://doi.org/10.1002/pbc.24096
Hinds, P.S., Yan, J., Gattuso, J.S., Hockenberry, M., Jones, H., Zupanec, S., . . . Srivastava, D.K. (2010). Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale—Child instrument. Journal of Pain and Symptom Management, 39, 572–578. https://doi.org/10.1016/j.jpainsymman.2009.07.015
Hockenberry, M.J., Hinds, P.S., Barrera P, Bryant, R., Adams-McNeill, J., Hooke, C., . . . Manteuffel, B. (2003). Three instruments to assess fatigue in children with cancer: The child, parent and staff perspectives. Journal of Pain and Symptom Management, 25, 319–328. https://doi.org/10.1016/S0885-3924(02)00680-2
Hockenberry, M.J., Hooke, M.C., McCarthy, K., & Gregurich, M.A. (2011). Sickness behavior clustering in children with cancer. Journal of Pediatric Oncology Nursing, 28, 263–272.
Hockenberry, M.J., Hooke, M.C., Rodgers, C., Taylor, O., Koerner, K.M., Mitby, P., . . . Pan, W. (2017). Symptom trajectories in children receiving treatment for leukemia: A latent class growth analysis with multitrajectory modeling. Journal of Pain and Symptom Management, 54, 1–8. https://doi.org/10.1016/j.jpainsymman.2017.03.002
Hockenberry, M.J., Taylor, O.A., Pasvogel, A., Rodgers, C., McCarthy, K., Gundy, P., . . . Moore, I.M. (2014). The influence of oxidative stress on symptom occurrence, severity, and distress during childhood leukemia treatment. Oncology Nursing Forum, 41, E238–E247. https://doi.org/10.1188/14.ONF.E238-E247
Hooke, M.C., Rodgers, C., Taylor, O., Koerner, K., Mitby, P., Moore, I, . . . Pan, W. (2018). Physical activity, the Childhood Cancer Symptom Cluster–Leukemia, and cognitive function: A longitudinal mediation analysis. Cancer Nursing, 41, 434–440. https://doi.org/10.1097/NCC.0000000000000634
Hu, L., & Bentler, P.M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. https://doi.org/10.1080/10705519909540118
Kestler, S.A., & LoBiondo-Wood, G. (2012). Review of symptom experiences in children and adolescents with cancer. Cancer Nursing, 35, E31–E49. https://doi.org/10.1097/NCC.0b013e3182207a2a
Kline, R.B. (2010). Principles and practice of structural equation modeling (3rd ed.). New York, NY: Guilford Press.
Kovacs, M. (2011). Children’s Depression Inventory 2TM. Toronto, ON, Canada: Multi-Health Systems Inc.
LeBourgeois, M.K., & Harsh, J.R. (2016). Development and psychometric evaluation of the Children’s Sleep-Wake Scale. Sleep Health, 2, 198–204. https://doi.org/10.1016/j.sleh.2016.04.001
Little, R.J.A. (1988). A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association, 83, 1198–1202. https://doi.org/10.2307/2290157
Lopes-Junior, L.C., Bomfim, E.O., Nascimento, L.C., Nunes, M.D., Pereira-da-Silva, B., & Lima, R.A. (2016). Non-pharmacological interventions to manage fatigue and psychological stress in children and adolescents with cancer: An integrative review. European Journal of Cancer Care, 25, 921–935. https://doi.org/10.1111/ecc.12381
Mandrell, B.N., Yang, J., Hooke, M.C., Wang, C., Gattuso, J.S., Hockenberry, M., . . . Hinds, P.S. (2011). Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. Journal of Pediatric Oncology Nursing, 28, 287–294. https://doi.org/10.1177/1043454211418667
Margolin, J.F., Rabin, K.R., Steuber, C.P., & Poplack, D.G. (2016). Acute lymphoblastic leukemia. In P.A. Pizzo, & D.G. Poplack (Eds.), Principles and practices of pediatric oncology (7th ed.). Philadelphia, PA: Lippincott Williams and Wilkins.
Meek, R., Kelly, A.M., & Hu, X.F. (2009). Use of the visual analog scale to rate and monitor severity of nausea in the emergency department. Academic Emergency Medicine, 16, 1304–1310. https://doi.org/10.1111/j.1553-2712.2009.00581.x
Mitchell, H.R., Lu, X., Myers, R.M., Sung, L., Balsamo, L.M., Carroll, W.L., . . . Kadan-Lottick, N. (2016). Prospective, longitudinal assessment of quality of life in children from diagnosis to 3 months off treatment for standard risk acute lymphoblastic leukemia: Results of Children’s Oncology Group study AALL0331. International Journal of Cancer, 138, 332–339. https://doi.org/10.1002/ijc.29708
Nunes, M.D.R., Bomfim, E.O., Olson, K., Lopes-Junior, L.C., Silva-Rodriques, F.M., Garcia de Lima, R.A., & Nascimento, L.C. (2018). Interventions minimizing fatigue in children/adolescents with cancer: An integrative review. Journal of Child Health Care, 22, 186–204. https://doi.org/10.1177/1367493517752498
Nunes, M.D.R., Jacob, E., Bomfim, E.O., Lopes-Junior, L.C., de Lima, R.A.G., Floria-Santos, M., & Nascimento, L.C. (2017). Fatigue and health related quality of life in children and adolescents with cancer. European Journal of Oncology Nursing, 29, 39–46. https://doi.org/10.1016/j.ejon.2017.05.001
Pan, H.T., Wu, L.M., & Wen, S.H. (2017). Quality of life and its predictors among children and adolescents with cancer. Cancer Nursing, 40, 343–351. https://doi.org/10.1097/NCC.0000000000000433
Rabin, K.R., Gramatges, M.M., Margolin, J.F., & Poplack, D.G. (2016). Acute lymphoblastic leukemia. In P.A. Pizzo PA, & D.G. Poplack (Eds.), Principles and practices of pediatric oncology (7th ed.). Philadelphia, PA: Lippincott Williams and Wilkins.
Rodgers, C., Hooke, M.C., Ward, J., & Linder, L.A. (2016). Symptom clusters in children and adolescents with cancer. Seminars in Oncology Nursing, 32, 394–404. https://doi.org/10.1016/j.soncn.2016.08.005
Scheurer, M.E., Lupo, P.J., & Bondy, M.L. (2016). Epidemiology of childhood cancer. In P.A. Pizzo, & D.G. Poplack (Eds.), Principles and practices of pediatric oncology (7th ed.). Philadelphia, PA: Lippincott Williams and Wilkins.
Sousa, K.H., Kwok, O.M., Schmiege, S.J., & West, S.G. (2014). A longitudinal approach to understanding the relationship between symptom status and QOL. Western Journal of Nursing Research, 36, 732–747. https://doi.org/10.1177/0193945913510980
Storfer-Isser, A., Lebourgeois, M.K., Harsh, J., Tompsett, C.J., & Redline, S. (2013). Psychometric properties of the adolescent sleep hygiene scale. Journal of Sleep Research, 22, 707–716. https://doi.org/10.1111/jsr.12059
Sung, L., Yanofsky, R., Klaassen, R.J., Dix, D., Pritchard, S., Winick, N., . . . Klassen, A. (2011). Quality of life during active treatment for pediatric acute lymphoblastic leukemia. International Journal of Cancer, 128, 1213–1120. https://doi.org/10.1002/ijc.25433
Tsze, D.S., von Baeyer, C.L., Pahalyants, V., & Dayan, P.S. (2018). Validity and reliability of the verbal numerical rating scale for children aged 4 to 17 years with acute pain. Annals of Emergency Medicine, 71, 691–702. https://doi.org/10.1016/j.annemergmed.2017.09.009
van Litsenburg, R.R., Huisman, J., Pieters, R., Verhaak, C., Kaspers, G.J., & Gemke, R.J. (2014). Determinants of quality of life during induction therapy in pediatric acute lymphoblastic leukemia. Supportive Care in Cancer, 22, 3235–3242. https://doi.org/10.1007/s00520-014-2349-2
Varni, J.W., Burwinkle, T.M., Katz, E.R., Meeske, K., & Dickinson, P. (2002). The PedsQL in pediatric cancer: Reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer, 94, 2090–2106.
Varni, J.W., Limbers, C., & Burwinkle, T.M. (2007). Literature review: Health-related quality of life measurement in pediatric oncology: Hearing the voices of the children. Journal of Pediatric Psychology, 32, 1151–1163. https://doi.org/10.1093/jpepsy/jsm008
Wood, C., von Baeyer, C.L., Falinower, S., Moyse, D., Annequin, D., & Legout, V. (2011). Electronic and paper versions of a faces pain intensity scale: Concordance and preference in hospitalized children. BMC Pediatrics, 11, 87. https://doi.org/10.1186/1471-2431-11-87
Woodgate, R.L. (2008). Feeling states: A new approach to understanding how children and adolescents with cancer experience symptoms. Cancer Nursing, 31, 229–238. https://doi.org/10.1097/01.NCC.0000305731.95839.ca




