Heart Rate Variability Markers as Correlates of Survival in Recipients of Hematopoietic Cell Transplantation
Objectives: To assess pre-/post-transplantation changes in autonomic tone, as measured by heart rate variability (HRV), among patients undergoing hematopoietic cell transplantation (HCT) and to look at those changes as they relate to post-transplantation survival rates.
Sample & Setting: Data were derived from a sample of 27 English-speaking patients undergoing allogeneic or autologous HCT at Stanford University.
Methods & Variables: A survival analysis using the Kaplan–Meier estimator was employed to explore whether increased HRV would enhance survival probabilities over time among patients undergoing HCT.
Results: An increased probability of survival was significantly related to increases in two HRV indexes: root mean square of successive differences and high frequency power.
Implications for Nursing: HRV may be a useful predictor of mortality among patients undergoing HCT. Interventions deliverable by nurses could be used to enhance HRV for patients identified as being at risk for early mortality.
Jump to a section
Hematologic cancer accounted for about 10% of all cancer deaths in the United States in 2017, and, on average, a person in the United States dies from hematologic cancer every nine minutes (Leukemia and Lymphoma Society, n.d.). For many malignant and nonmalignant hematologic diseases, the treatment of choice is hematopoietic cell transplantation (HCT). HCT has become a widely used, effective treatment, which has significantly increased overall survival rates for many patients (Bhatia et al., 2005, 2007; Socié et al., 1999). The Center for International Bone and Marrow Transplant Research estimated that in the United States, more than 20,000 individuals received HCT (allogeneic and autologous) in 2015, and this number continues to grow (D’Souza & Zhu, 2016). For some patients, a transplantation is key for longevity. However, the underlying mechanisms that predict mortality or survival following transplantation are not fully understood.
Conventional medical and demographic pretransplantation risk factors that have been found to predict mortality in this patient population include age, disease status, Karnofsky Performance Status score (Sorror et al., 2008), relapse following transplantation, and the Hematopoietic Cell Transplantation–Comorbidity Index score (Sorrer et al., 2005, 2008). However, to the current authors’ knowledge, researchers examining mortality among patients undergoing HCT have not studied the impact of autonomic nervous system functioning, which has been implicated in the progression of malignancies. One way of measuring autonomic functioning is through electrocardiogram assessment of heart rate variability (HRV). HRV specifically assesses the oscillations in the beat-to-beat intervals of consecutive heartbeats measured over a fixed time interval. Power spectral analysis of these heart rate fluctuations allows for evaluation of cardiovascular control by the sympathetic and parasympathetic branches of the autonomic nervous system (Akselrod et al., 1981; Chapleau & Sabharwal, 2011).
HRV is assessed using time and frequency domain analysis. Time domain analysis focuses on the length of time between normal-to-normal (NN) heartbeat intervals (i.e., the length of time between R waves on adjacent QRS complexes in a continuous electrocardiogram record). When plotted, the variation of these NN intervals over time forms the HRV wave. Time domain measures include the standard deviation of NN R-R intervals (SDNN) and the root mean square of successive differences (rMSSD), which is the square root of the mean squared differences of successive NN intervals. The SDNN and the rMSSD are considered to be global measures of HRV, with the SDNN reflecting sympathetic and parasympathetic activity responsible for variability during the recording period and the rMSSD being more closely tied to parasympathetic activity.
HRV frequency domain measures are obtained through power spectral analysis of the HRV wave. This allows for evaluation of cardiovascular control by the sympathetic and parasympathetic branches of the autonomic nervous system. Frequency domain measures include high frequency power (HF), low frequency power (LF), and the ratio of low frequency power to high frequency power (LF/HF). HF is mediated solely by the vagus nerve, which is the main conduit of the parasympathetic nervous system; HF elevations suggest greater parasympathetic control of the heart. LF tends to be more often linked with sympathetic nervous system activity. However, whether LF represents a mixture of activation within both autonomic branches has been controversial. The frequency domain LF/HF is typically used to assess the balance of sympathetic to parasympathetic nervous system control (Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology, 1996a, 1996b).
Lower SDNN has been found to be predictive of mortality in those with prostate and non-small cell lung cancer (De Couck, van Brummelen, Schallier, De Grève, & Gidron, 2013). Kim et al. (2010) found similar results among terminally ill patients with cancer in hospice, even after adjusting for demographic and other clinically meaningful factors. In addition, lower HF was significantly predictive of shorter survival length among patients in hospice with hepatocellular carcinoma (Chiang, Koo, Kuo, & Fu, 2010), as well as among individuals with breast cancer (Palesh et al., 2008).
Increasing evidence supports the implication of autonomic nervous system functioning in the proliferation of malignant cancer cells. The autonomic nervous system modulates the body’s immune system, which is responsible for regulating inflammation. When the immune system becomes dysregulated, inflammation accumulates, resulting in an environment conducive to the initiation and growth of malignant tumor cells. As a result, examining the connection among the autonomic nervous system, the immune system, and chronic inflammation is of the utmost importance in understanding cancer proliferation.
The specific link between the autonomic nervous system and the immune system is through an anti-inflammatory circuit, the inflammatory reflex, surrounding the vagus nerve, which is an important component of the parasympathetic nervous system (Rosas-Ballina et al., 2011; Rosas-Ballina & Tracey, 2009; Tracey, 2002). The inflammatory reflex works by sensing the activity of proinflammatory cytokines through an afferent pathway (Olofsson, Rosas-Ballina, Levine, & Tracey, 2012) and informs the brain of malignancies. An efferent response is then elicited, in which signals from the brain, via the vagus nerve, stimulate acetylcholine-producing T cells. In turn, the T cells activate anti-inflammation processes by inhibiting proinflammatory cytokine production (Olofsson et al., 2012; Rosas-Ballina et al., 2011). The vagus nerve affects the maturation and reduction of cancerous tumor cells because of its role in immune system signaling and regulating (De Couck et al., 2013; De Couck, Mravec, & Gidron, 2012; Levy, Herberman, Lippman, D’Angelo, & Lee, 1991; Mravec, Gidron, & Hulin, 2008; Rosas-Ballina & Tracey, 2009).
The theoretical underpinning of this study rests on the close relationship between the autonomic nervous system and the immune system. Measuring sympathetic and parasympathetic activity is relevant to cancer development because of its role in immune regulation; it may provide additional insight into longevity and survival in populations with cancer. In addition, autonomic nervous system function may be particularly important in patients undergoing HCT, given the immune dysregulation that occurs with HCT in the context of graft-versus-lymphoma or graft-versus-leukemia effects on disease relapse. This research hypothesizes a relationship between HRV as an indicator of autonomic nervous system functioning and survival in people with cancer.
The aim of this study is to assess changes in autonomic tone, as measured by HRV, in a sample of patients undergoing HCT, before and after transplantation, and to examine these changes in relation to survival rates post-transplantation. This is a secondary analysis of data from a parent study that investigated the efficacy of a novel insomnia intervention in this sample of patients undergoing HCT. As a result, the patients undergoing HCT also experienced insomnia, a condition that has been closely linked with decreased HRV. The current authors hypothesized that additional decreases in HRV over time, which suggest a dysregulation in autonomic nervous system functioning, would be associated with shorter survival in this patient population. Evidence supporting this hypothesis would offer prognostic information to treating providers. Nurses could measure HRV indexes and perhaps even deliver interventions (e.g., biofeedback, other relaxation techniques) (Lehrer & Gevirtz, 2014).
Methods
The data for this study were drawn from a parent study that examined the feasibility and efficacy of using a novel intervention called Brief Behavioral Therapy for Insomnia (BBT-I) in patients undergoing HCT as part of a randomized, clinical trial. Because no significant group differences were noted between the BBT-I group and the control group regarding symptoms of insomnia after delivery of the BBT-I intervention, there was no need to separate the two groups. Consequently, this study was based on the full sample of 21 evaluable participants.
Sample and Setting
The appropriate institutional review board at Stanford University approved this study, and all participants gave written informed consent. Data were derived from a sample of 27 English-speaking patients undergoing allogeneic or autologous HCT who experienced insomnia, as indicated by a score of 8 or greater on the Insomnia Severity Index (Morin, Belleville, Bélanger, & Ivers, 2011). The participants were stratified by whether they received allogeneic versus autologous HCT and then randomized to receive either the BBT-I intervention (two face-to-face sessions and four telephone calls during a six-week period) or a waitlist control condition (participants assigned to a waiting list received the intervention after the study was completed).
Data were collected at two time points: (a) baseline visit pretransplantation and (b) follow-up visit post-transplantation. Patients were excluded if they had a history of uncontrolled psychiatric disorders, if they were pregnant, or if they had preexisting chronic insomnia or other sleep disorders. Patients selected for inclusion were those who had received a transplantation at the study site (Stanford University), were aged 21 years or older, had scored at least an 8 on the Insomnia Severity Index, and did not have surgery scheduled within the study period. Participants were referred by study site physicians, and they were screened for inclusion or exclusion by research staff during a clinic visit.
Of the 127 patients who were seen at the study site for either consultation or HCT from March 2012 to July 2013, 27 were eligible and were enrolled. Reasons provided for exclusion included the patient’s absence of insomnia, non-English–speaking ability, transplantation having already taken place, suffering from another sleep disorder, and not having the time for or interest in the study, as well as physician report that the patient was too sick. Of the 27 eligible participants, 21 completed pre-/post-transplantation study visits.
Measures
Information on participants’ age, gender, and ethnicity were collected using a demographics questionnaire. The following variables were extracted from medical records:
• Cancer diagnosis
• Disease status
• Transplantation type (allogeneic or autologous)
• Relapse after engraftment
• Hematopoietic Cell Transplantation–Comorbidity Index score
• Treatment type
• Karnofsky Performance Status score
Participants completed a number of subjective and objective symptom scales.
Autonomic Nervous System Activity
The Firstbeat® device was used to measure HRV for as long as 60 minutes at baseline and at the follow-up visit. The device measures R-R intervals with a sample rate of 1 ms. The following time and frequency domain indexes were calculated:
• Time measures: (a) SDNN and (b) rMSSD; both measures are representative of total variability in heart rate.
• Frequency measures: (a) HF (natural log) and (b) LF/HF (Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology, 1996a, 1996b); each participant’s mean heart rate was also measured.
The LF scores were not used in the analyses for this study because the validity of this measure used alone has been called into question in the literature (Eckberg, 1997).
Survival Outcome
The survival analysis was performed using the time of survival since transplantation versus all-cause death since the HCT transplantation. In this analysis, participants who were still alive by the end of the study period (February 2016) were censored. (Censoring is part of the standard procedure for survival analysis and is necessary to complete the analysis. Data of participants still alive at the conclusion of the study are censored because information about survival time is incomplete.) Total follow-up time was 16 months. The Kaplan–Meier survival curves were computed, and the log-rank test was used. The a priori significance level was set at 0.05.
Statistical Analysis
The numeric variables characterizing the changes in HRV measures (SDNN, rMSSD, HF, and LF/HF) and in mean heart rate over time were dichotomized at zero. Change values of zero or less indicated no change or a decrease over time in the measures of HRV and mean heart rate. Change values greater than zero indicated an increase over time in the measures of HRV and mean heart rate. These cutoff points were determined by clinical standards. With this approach, clinicians can easily interpret risk by tracking whether HRV increases or decreases over time. The Kaplan–Meier survival curves were used to estimate the survival curve for the various strata of HRV measures. As a secondary analysis, using the Kaplan–Meier estimator, the current authors explored the survival curves by using other medical variables relevant to survival, based on previous research findings (Davies et al., 2000; Michelis et al., 2013; Sorror et al., 2008). IBM SPSS Statistics, version 22.0, was used to conduct the analyses.
Results
The current authors analyzed the data of 21 patients who underwent HCT. Participants were aged 27–70 years. Thirteen participants had received an autologous HCT, and eight had received an allogeneic HCT. In patients for whom engraftment was successful, the average length of engraftment of neutrophils was 10 days, and the average length of engraftment of platelets was 17 days. The median survival time since transplantation was 151 days. Additional demographic and medical characteristics of the participants are summarized in Table 1. 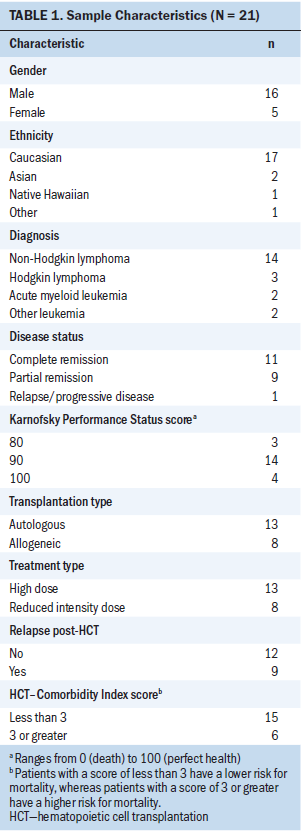
Table 2 presents the means and standard deviations of the pre-/post-transplantation HRV measures. As expected, all HRV values of patients who had undergone HCT were significantly lower than those of the healthy controls (p < 0.001) (Nunan, Sandercock, & Brodie, 2010) but were comparable to the values found in other populations with cancer (De Couck & Gidron, 2013; Kim et al., 2010). 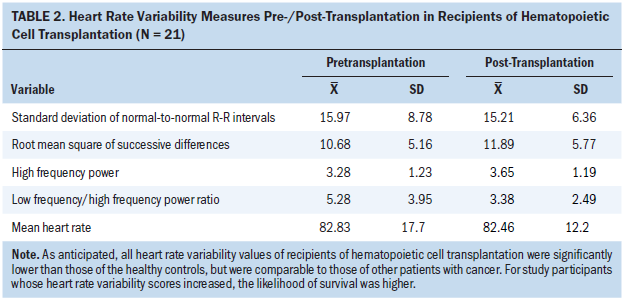
Follow-up data were available until February 2016, by which time five individuals had died: three patients died from persistent disease, one died from graft-versus-host disease, and one died from a bacterial infection. Figure 1 shows the Kaplan–Meier survival curve for the total group. Overall survival probability for the full cohort was greater than 85% at one-year follow-up and greater than 80% at two years after HCT. 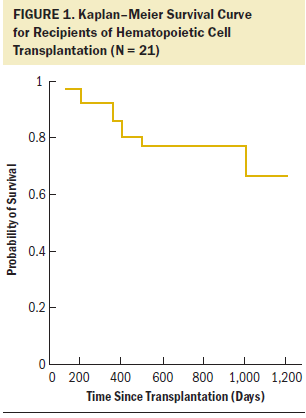
The dichotomized indexes of the HRV measures (SDNN, rMSSD, HF, and LF/HF) were used to examine the group differences in relation to the survival curves. Log-rank tests of the equality of the group survival distributions showed that significant differences in survival existed between groups for rMSSD (c = 4.087, p = 0.043) and HF strata (c = 10.419, p = 0.001). Those results support the hypothesis that the relative risk of death was significantly reduced for patients whose HRV increased from pre- to post-transplantation. Figures 2 and 3 show Kaplan–Meier cumulative survival curves for rMSSD and HF. Patients whose rMSSD score increased over time had a two-year survival probability of 90% compared to that of 70% in patients whose rMSSD score decreased. The differences in cumulative survival for HF scores were even larger, with patients whose scores increased over time having a survival probability of 100% at two years post-transplantation versus that of 50% in patients with a decreased HF score. As for the other dichotomized HRV measures, SDNN and LF/HF and mean heart rate were not significantly associated with mortality in this patient population; however, SDNN was trending in the expected direction (c = 2.618, p = 0.106).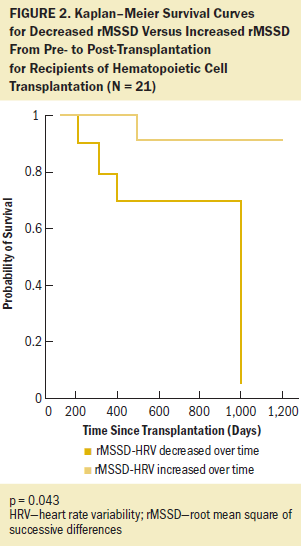
The only other predictor variable that was significantly related to survival was age when dichotomized by the median of 58 years of age (c = 6.252, p = 0.012). A comparison of Kaplan–Meier survival curves indicated that gender (c = 0.005, p = 0.944), relapse post-transplantation (c = 2.909, p = 0.088), transplantation type (c = 0.004, p = 0.95), Hematopoietic Cell Transplantation–Comorbidity Index score (c = 0.67, p = 0.413) (Sorror et al., 2005, 2008), disease status (c = 0.427, p = 0.513), and Karnofsky Performance Status score (c = 1.203, p = 0.273) were not found to be significantly related to survival in this sample, likely because of the small sample size. 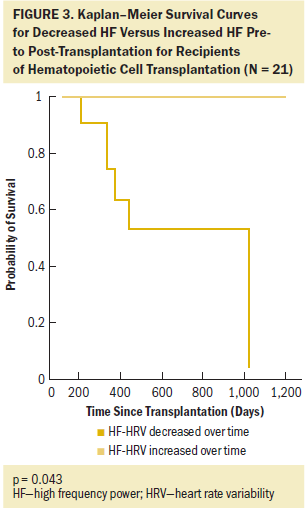
Discussion
Results of the current study suggest that increases in HRV over time were correlated with survival in patients who underwent HCT. These findings are clinically significant because HRV is known to respond to behavioral and pharmacologic interventions, whereas other known demographic and medical risk factors in this patient population are nonmodifiable. The two HRV measures found to significantly predict longevity were increases in rMSSD and HF. The SDNN was trending in the expected direction. This study’s results extend previous research findings showing that greater HRV is associated with longer survival in other populations of individuals with cancer (Chiang et al., 2010; Giese-Davis et al., 2015; Kim et al., 2010).
These findings point to the theoretical and clinical importance of HRV markers and, in particular, to HF and rMSSD as indicators of longevity in patients undergoing HCT. HF and rMSSD are the two recommended markers of parasympathetic nervous system activity and vagal tone, according to current standards of measurement (Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology, 1996a, 1996b). Evidence has emerged to support the idea that the vagus nerve plays an important role in immune functioning via the inflammatory reflex (Tracey, 2002). Dysregulation in this reflex system, as may be indicated by low HF and rMSSD measures, is associated with increased inflammation, which, in turn, is implicated in the pathogenesis of cancer growth and initiation (De Couck et al., 2013). Stimulation of this inflammatory reflex via electrical inputs or selective drugs has been shown to result in reduced inflammation and enhanced likelihood of survival in models of hemorrhagic shock (Guarini et al., 2003), sepsis (Wang et al., 2004), and pancreatitis (van Westerloo et al., 2006). As expected, the HRV markers in patients undergoing HCT in the current study were disproportionally low compared to those in healthy adult populations (Nunan et al., 2010). The lower HRV scores found in this study are consistent with scores found in other populations of individuals with cancer (Kim et al., 2010). Findings of the current study suggest that, despite the low HRV markers, increases in rMSSD and HF over time were associated with a significant increase in survival in this population.
Limitations
Findings are limited to patients undergoing HCT with insomnia. This study was based on a small sample, which likely explains why none of the conventional risk factors significantly predicted mortality, with the exception of age. However, the significant findings in HRV measures suggest that the relationships between increases in HF and rMSSD and probability of survival were robust. In addition, the restriction of the sample to patients with insomnia enhanced the statistical power of testing the hypothesis in the small sample by reducing extraneous variability among the participants.
Implications for Nursing
Many of the traditional demographic and medical risk factors associated with bone marrow transplantation are either non-modifiable or difficult-to-manage medical comorbidities or complications. HRV, on the other hand, may be modifiable. Assessment of HRV could be incorporated into the standard-of-care protocol during medical visits by nurses and other healthcare professionals. HRV is easily assessed with inexpensive portable devices. Nurses could help to track HRV markers before and after transplantation for each patient. Working in close collaboration with the treating oncologist, nurses could help to identify patients at risk for decreased HRV. Nurses could also provide patients with information and education on HRV and on the relationship between physical health and autonomic nervous system functioning. Although the current authors recognize that this is an idealistic goal, given the high work demands and time constraints of nurses in today’s healthcare systems, trained nurses working in a clinic could potentially deliver interventions or point patients to resources that may help to improve HRV. Interventions could include not only drugs and electrical stimulation but also behavioral interventions that can be learned, such as HRV biofeedback (Lehrer & Gevirtz, 2014).
HRV biofeedback is a process by which patients receive instant feedback on their heart rhythm and breathing rate (Lehrer & Gevirtz, 2014). Prior research has indicated several possible mechanisms of how HRV biofeedback may affect the parasympathetic nervous system (Gevirtz, 2013; Lehrer & Gevirtz, 2014) as it helps to restore autonomic homeostasis, vagal afferent pathways, and the vagal inflammation reflex system (Gevirtz, 2013). A randomized, controlled trial by O’Rourke et al. (2017) found that cancer survivors who received weekly HRV biofeedback training for six weeks experienced improved HRV, which, in turn, also had a positive impact on symptoms of pain, depression, stress, and fatigue. Randomized, controlled trials by Kern-Buell, McGrady, Conran, and Nelson (2000) and McGrady et al. (1992) have shown HRV biofeedback effectiveness in improving immune functioning, as indicated by a reduced number of cytokines in the blood. Another randomized, controlled trial by Lehrer et al. (2010) did not discover effects on proinflammatory cytokines following an HRV biofeedback intervention in a sample of 11 individuals exposed to lipopolysaccharide (inflammatory condition); however, the researchers found improvements in autonomic dysfunction following the HRV biofeedback intervention.
If nurses teach patients HRV biofeedback and other relaxation techniques (e.g., diaphragmatic breathing), patients may be able to enhance their parasympathetic activity and vagal tone, potentially improving immune functioning. DeMarco-Sinatra (2000) reviewed the benefits of incorporating different types of relaxation interventions into nursing practice and highlighted relaxation training as an effective auxiliary intervention tool for a number of medical populations, including patients with cancer. Nurses can be trained to deliver relaxation interventions in the clinic during medical visits. In addition, if necessary, nurses could help to make appropriate referrals to biofeedback specialists or mental health counselors for the treatment and delivery of more involved intervention modalities. More research involving patients with cancer in this area is needed to examine the use of HRV biofeedback and other relaxation techniques as potential tools that may help to enhance immune functioning and nurses’ ability to administer such interventions to patients identified as at risk for low HRV.
Conclusion
The current study provides evidence that improvements in HRV (specifically rMSSD and HF) correlate with survival in patients who have undergone HCT. Potential mechanisms that may explain this relationship relate to the vagus nerve, which signals the presence of proinflammatory cytokines and activates anti-inflammatory processes. If the vagus nerve does not function sufficiently, which may be the case in patients with low HRV, inflammation can increase and affect the maturation of cancerous tumor cells. This study’s findings highlight the need for additional research with patients undergoing HCT to examine the effects of behavioral treatments delivered by nurses and other healthcare professionals on HRV and survival.
The authors gratefully acknowledge Cheryl Koopman, PhD, and Jane Paik Kim, PhD, for serving as consultants for statistical analysis and for reviewing and editing the methods and results sections, as well as their laboratory team members at the Stanford University Cancer Survivorship Research Laboratory, Bingjie Tong, BS, and Bailey Selland, MS, for their contributions to the manuscript, including article review and proofreading.
About the Author(s)
Caroline Scheiber, PhD, is a postdoctoral fellow in the School of Medicine, Department of Psychiatry and Behavioral Sciences, Laura Johnston, MD, is an associate professor in the School of Medicine, Division of Blood and Marrow Transplantation, and Mary Melissa Packer, MA, is a lab manager in the School of Medicine, Department of Psychiatry and Behavioral Sciences, all at Stanford University in California; Richard Gevirtz, PhD, is a distinguished professor in the California School of Professional Psychology at Alliant International University in San Diego; and Katharine S. Edwards, PhD, is a clinical assistant professor in the School of Medicine, Department of Cardiovascular Medicine, and Oxana Palesh, PhD, MPH, is an assistant professor in the School of Medicine, Department of Psychiatry and Behavioral Sciences, both at Stanford University in California. This research was funded by a grant (NCT01536977) from the National Cancer Institute and the Stanford Cancer Institute at Stanford University School of Medicine. Palesh has previously consulted for the Substance Abuse and Mental Health Services Administration Center for Substance Abuse Treatment. Johnston, Gevirtz, Scheiber, and Palesh contributed to the conceptualization. Johnston and Palesh contributed to the design. Palesh and Johnston completed the data collection. Scheiber, Gevirtz, and Palesh provided statistical support and the analysis. Scheiber, Johnston, Packer, Edwards, and Palesh contributed to the manuscript preparation. Scheiber can be reached at carolinescheiber@gmail.com, with copy to ONFEditor@ons.org. (Submitted July 2017. Accepted October 10, 2017.)
References
Akselrod, S., Gordon, D., Ubel, F.A., Shannon, D.C., Berger, A.C., & Cohen, R.J. (1981). Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science, 213, 220–222.
Bhatia, S., Francisco, L., Carter, A., Sun, C.-L., Baker, K.S., Gurney, J.G., . . . Weisdorf, D.J. (2007). Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood, 110, 3784–3792. https://doi.org/10.1182/blood-2007-03-082933
Bhatia, S., Robison, L.L., Francisco, L., Carter, A., Liu, Y., Grant, M., . . . Forman, S.J. (2005). Late mortality in survivors of autologous hematopoietic-cell transplantation: Report from the Bone Marrow Transplant Survivor Study. Blood, 105, 4215–4222. https://doi.org/10.1182/blood-2005-01-0035
Chapleau, M.W., & Sabharwal, R. (2011). Methods of assessing vagus nerve activity and reflexes. Heart Failure Reviews, 16, 109–127. https://doi.org/10.1007/s10741-010-9174-6
Chiang, J.-K., Koo, M., Kuo, T.B., & Fu, C.-H. (2010). Association between cardiovascular autonomic functions and time to death in patients with terminal hepatocellular carcinoma. Journal of Pain and Symptom Management, 39, 673–679. https://doi.org/10.1016/j.jpainsymman.2009.09.014
Davies, S.M., Kollman, C., Anasetti, C., Antin, J.H., Gajewski, J., Casper, J.T., . . . Kernan, N.A. (2000). Engraftment and survival after unrelated-donor bone marrow transplantation: A report from the National Marrow Donor Program. Blood, 96, 4096–4102.
De Couck, M., & Gidron, Y. (2013). Norms of vagal nerve activity, indexed by heart rate variability, in cancer patients. Cancer Epidemiology, 37, 737–741. https://doi.org/10.1016/j.canep.2013.04.016
De Couck, M., Mravec, B., & Gidron, Y. (2012). You may need the vagus nerve to understand pathophysiology and to treat diseases. Clinical Science, 122, 323–328. https://doi.org/10.1042/CS20110299
De Couck, M., van Brummelen, D., Schallier, D., De Grève, J., & Gidron, Y. (2013). The relationship between vagal nerve activity and clinical outcomes in prostate and non-small cell lung cancer patients. Oncology Reports, 30, 2435–2441. https://doi.org/10.3892/or.2013.2725
DeMarco-Sinatra, J. (2000). Relaxation training as a holistic nursing intervention. Holistic Nursing Practice, 14(3), 30–39.
D’Souza, A., & Zhu, X. (2016). Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2016. Retrieved from https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/page…
Eckberg, D.L. (1997). Sympathovagal balance: A critical appraisal. Circulation, 96, 3224–3232.
Gevirtz, R. (2013). The promise of heart rate variability biofeedback: Evidence-based applications. Biofeedback, 41, 110–120. https://doi.org/10.5298/1081-5937-41.3.01
Giese-Davis, J., Wilhelm, F.H., Tamagawa, R., Palesh, O., Neri, E., Taylor, C.B., . . . Spiegel, D. (2015). Higher vagal activity as related to survival in patients with advanced breast cancer: An analysis of autonomic dysregulation. Psychosomatic Medicine, 77, 346–355. https://doi.org/10.1097/PSY.0000000000000167
Guarini, S., Altavilla, D., Cainazzo, M.M., Giuliani, D., Bigiani, A., Marini, H., . . . Squadrito, F. (2003). Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation, 107, 1189–1194.
Kern-Buell, C.L., McGrady, A.V., Conran, P.B., & Nelson, L.A. (2000). Asthma severity, psychophysiological indicators of arousal, and immune function in asthma patients undergoing biofeedback-assisted relaxation. Applied Psychophysiology and Biofeedback, 25, 79–91.
Kim, D.H., Kim, J.A., Choi, Y.S., Kim, S.H., Lee, J.Y., & Kim, Y.E. (2010). Heart rate variability and length of survival in hospice cancer patients. Journal of Korean Medical Science, 25, 1140–1145. https://doi.org/10.3346/jkms.2010.25.8.1140
Lehrer, P., Karavidas, M.K., Lu, S.-E., Coyle, S.M., Oikawa, L.O., Macor, M., . . . Lowry, S.F. (2010). Voluntarily produced increases in heart rate variability modulate autonomic effects of endotoxin induced systemic inflammation: An exploratory study. Applied Psychophysiology and Biofeedback, 35, 303–315. https://doi.org/10.1007/s10484-010-9139-5
Lehrer, P.M., & Gevirtz, R. (2014). Heart rate variability biofeedback: How and why does it work? Frontiers in Psychology, 5. https://doi.org/10.3389/fpsyg.2014.00756
Leukemia and Lymphoma Society. (n.d.). Facts and statistics. Retrieved from https://www.lls.org/http%3A/llsorg.prod.acquia-sites.com/facts-and-stat…
Levy, S.M., Herberman, R.B., Lippman, M., D’Angelo, T., & Lee, J. (1991). Immunological and psychosocial predictors of disease recurrence in patients with early-stage breast cancer. Behavioral Medicine, 17, 67–75. https://doi.org/10.1080/08964289.1991.9935161
McGrady, A., Conran, P., Dickey, D., Garman, D., Farris, E., & Schumann-Brzezinski, C. (1992). The effects of biofeedback-assisted relaxation on cell-mediated immunity, cortisol, and white blood cell count in healthy adult subjects. Journal of Behavioral Medicine, 15, 343–354.
Michelis, F.V., Atenafu, E.G., Gupta, V., Kim, D.D., Kuruvilla, J., Lambie, A., . . . Messner, H.A. (2013). Duration of first remission, hematopoietic cell transplantation-specific comorbidity index and patient age predict survival of patients with AML transplanted in second CR. Bone Marrow Transplantation, 48, 1450–1455. https://doi.org/10.1038/bmt.2013.71
Morin, C.M., Belleville, G., Bélanger, L., & Ivers, H. (2011). The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608.
Mravec, B., Gidron, Y., & Hulin, I. (2008). Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Seminars in Cancer Biology, 18, 150–163. https://doi.org/10.1016/j.semcancer.2007.12.002
Nunan, D., Sandercock, G.R., & Brodie, D.A. (2010). A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing and Clinical Electrophysiology, 33, 1407–1417. https://doi.org/10.1111/j.1540-8159.2010.02841.x
Olofsson, P.S., Rosas-Ballina, M., Levine, Y.A., & Tracey, K.J. (2012). Rethinking inflammation: Neural circuits in the regulation of immunity. Immunological Reviews, 248, 188–204. https://doi.org/10.1111/j.1600-065X.2012.01138.x
O’Rourke, M.A., Stokes, S., Regina, F., Susko, K., Hendry, W., Anderson, A., . . . Burch, J. (2017). Heart rate variability (HRV) training for symptom control in cancer survivors [Abstract 148]. Journal of Clinical Oncology, 35(Suppl. 5), 148. https://doi.org/10.1200/JCO.2017.35.5_suppl.148
Palesh, O., Zeitzer, J.M., Conrad, A., Giese-Davis, J., Mustian, K.M., Popek, V., . . . Spiegel, D. (2008). Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. Journal of Clinical Sleep Medicine, 4, 441–449.
Rosas-Ballina, M., Olofsson, P.S., Ochani, M., Valdés-Ferrer, S.I., Levine, Y.A., Reardon, C., . . . Tracey, K.J. (2011). Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science, 334, 98–101. https://doi.org/10.1126/science.1209985
Rosas-Ballina, M., & Tracey, K.J. (2009). The neurology of the immune system: Neural reflexes regulate immunity. Neuron, 64, 28–32. https://doi.org/10.1016/j.neuron.2009.09.039
Socié, G., Stone, J.V., Wingard, J.R., Weisdorf, D., Henslee-Downey, P.J., Bredeson, C., . . . Klein, J.P. (1999). Long-term survival and late deaths after allogeneic bone marrow transplantation. New England Journal of Medicine, 341, 14–21. https://doi.org/10.1056/NEJM199907013410103
Sorror, M., Storer, B., Sandmaier, B.M., Maloney, D.G., Chauncey, T.R., Langston, A., . . . Storb, R. (2008). Hematopoietic Cell Transplantation-Comorbidity Index and Karnofsky Performance Status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer, 112, 1992–2001. https://doi.org/10.1002/cncr.23375
Sorror, M.L., Maris, M.B., Storb, R., Baron, F., Sandmaier, B.M., Maloney, D.G., & Storer, B. (2005). Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood, 106, 2912–2919. https://doi.org/10.1182/blood-2005-05-2004
Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology. (1996a). Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation, 93, 1043–1065.
Task Force of the European Society of Cardiology & North American Society of Pacing and Electrophysiology. (1996b). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17, 354–381.
Tracey, K.J. (2002). The inflammatory reflex. Nature, 420, 853–859. https://doi.org/10.1038/nature01321
van Westerloo, D.J., Giebelen, I.A., Florquin, S., Bruno, M.J., LaRosa, G.J., Ulloa, L., . . . van der Poll, T. (2006). The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology, 130, 1822–1830. https://doi.org/10.1053/j.gastro.2006.02.022
Wang, H., Liao, H., Ochani, M., Justiniani, M., Lin, X., Yang, L., . . . Ulloa, L. (2004). Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Medicine, 10, 1216–1221. https://doi.org/10.1038/nm1124




