Effects of Peer-Led Interventions for Patients With Cancer: A Meta-Analysis
Problem Identification: To evaluate the effects of peer-led supportive interventions for patients with cancer.
Literature Search: Six electronic databases (EMBASE, MEDLINE®, Google Scholar, Cochrane Library, ProQuest Medical Library, and CINAHL®) were searched for articles published from 1997 to May 2017.
Data Evaluation: A total of 159 studies were identified. Eighteen (16 randomized, controlled trials [RCTs] and 2 non-RCTs) were eligible for systematic review and 16 for meta-analysis. The Cochrane risk of bias tool and Comprehensive Meta-Analysis software were used for analysis.
Synthesis: The authors synthesized the results of the effect size of each trial according to cancer symptoms, coping, emotional health, quality of life, self-efficacy, sexuality, social support, and health-related behaviors.
Implications for Research: The findings from this study suggest that an additional tiered evaluation that has a theoretical underpinning and high-quality methodology is required to confirm the efficacy of peer-led supportive interventions within cancer care models.
Jump to a section
The earlier detection and treatment of many types of cancer has significantly extended the life expectancies of patients during the past two decades (Siegel, Miller, & Jemal, 2015). However, cancer and its treatment can lead to physical disability, emotional distress, and social problems. Even after treatment, a cancer survivor often requires care from multiple providers to manage the long-term sequelae of the illness and treatment. Patients with cancer who have prolonged survival times often have unmet supportive care needs (Hodgkinson, Butow, Hobbs, & Wain, 2007).
Patients with cancer who have less social support during and after treatment are more likely to experience distress (Andrykowski, Lykins, & Floyd, 2008). Social support can contribute to general well-being and buffer the impact of stressful experiences, including those related to life-threatening illnesses (Cohen & Wills, 1985). Peer support is a common form of social support because it provides patients with opportunities for experiential empathy. Peer-led supportive interventions (PSIs), in which individuals communicate and share experiences with others who have had similar personal experiences, can help to build self-efficacy, or the belief that one is capable of performing a course of action to reach a desired goal (Bandura, 1997). Self-efficacy is key to an individual’s successful self-management of diverse chronic illnesses and, therefore, helps to improve health outcomes (Lorig & Holman, 2003). In recognition of the importance of social relationships and support from peers, intimate partners, or family members, experiential knowledge has become significant in the delivery of quality health care (Cox, 1993; Eng & Young, 1992).
Numerous studies of PSIs in the past 20 years have examined their effects on physical problems, psychosocial distress, unhealthy behaviors, and coping skills. However, these studies have had discordant results, and many have not satisfactorily met the outcome expectations. For example, previous trials in which the intimate partner or family members participated as care providers showed inconsistent effects on sexuality (Bultz, Speca, Brasher, Geggie, & Page, 2000; Campbell et al., 2007; Porter et al., 2009). Studies in which breast cancer survivors who completed primary treatment provided other patients with breast cancer with supportive interventions had inconsistent results regarding quality of life (QOL), emotional health, and self-efficacy (Giese-Davis et al., 2016; Lee, Lee, Oh, & Kim, 2013; Napoles et al., 2015; Wittenberg et al., 2010). In addition, PSIs that employed active listening or sharing of experiences regarding the emotional health of patients have had inconsistent results (Crane-Okada et al., 2012; Giese-Davis et al., 2016; Lee et al., 2013; Weber et al., 2004; Weber, Roberts, Yarandi, Mills, Chumbler, & Algood, 2007; Weber, Roberts, Yarandi, Mills, Chumbler, & Wajsman, 2007). The discordance among these previous studies suggests that PSIs may provide no clear benefit in facilitating psychological adjustment or in aiding recovery from the traumatic experiences of cancer. The conclusions of these previous studies may differ as a result of heterogeneity in application of theory, control conditions, study design, characteristics of peers, or intensity of the intervention. Therefore, a more thorough synthesis of these studies is needed to draw an integrated conclusion on the effects of PSIs and to identify reasons for the varying results.
Campbell, Phaneuf, and Deane (2004) reviewed 21 studies on PSIs (18 nonexperimental studies and 3 randomized, controlled trials [RCTs]). Hoey, Ieropoli, White, and Jefford (2008) also reviewed 43 studies on PSIs for patients with cancer (35 nonexperimental studies and 8 RCTs). However, both reviews included diverse models of peer-led support (one-on-one peer support, group-based therapy without one-on-one support, and Internet-based peer support). Macvean, White, and Sanson-Fisher (2008) reviewed 28 nonexperimental studies and 4 RCTs that examined one-on-one support programs using volunteers; this review included homogeneous peer-led models, such as one-on-one support, but most of the RCTs were published before 1996, and the authors did not show the combined effect sizes (ESs) of the trials. These reviews (Campbell et al., 2004; Hoey et al., 2008; Macvean et al., 2008) concluded that benefits stemmed from peer support, as well as that contact with other cancer survivors provides practical, social, and emotional support. However, this conclusion is not certain because these studies used heterogeneous models of peer-led support, did not analyze ESs, and relied on data collected more than 20 years ago. In addition, these previous reviews (Campbell et al., 2004; Hoey et al., 2008; Macvean et al., 2008) did not formally assess study heterogeneity. The small number of RCTs included in these previous reviews is also a limitation.
Performing a meta-analysis to determine the effects of a specific model of peer support (one-on-one or face-to-face peer-led supportive care) is necessary. The current meta-analysis examines previous trials to quantify the ESs of different PSIs on various outcomes of patients with cancer.
Methods
The review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Liberati et al., 2009). All included studies were RCTs or non-RCTs that examined the effects of supportive interventions led by the peers or partners of adults aged 18 years or older who had been diagnosed with cancer; these interventions were compared with healthcare professional–led care or usual care.
Data Sources and Searches
The search strategy was developed in collaboration with an experienced research librarian. Articles were identified through searches of MEDLINE®, EMBASE, Google Scholar, Cochrane Library, ProQuest Medical Library, and CINAHL® without search limits. Manual reviews of reference lists from publications identified in these databases were also performed. All searches included studies published in English from 1997 to May 2017. All RCTs and non-RCTs of PSIs for patients with cancer were included. The search terms were as follows, with the syntax modified according to the guidelines of each database: (“Neoplasms” OR “Cancer”) AND (“Peer” OR “Mentor” OR “Volunteer” OR “Lay” OR “Partner” OR “Supporter” OR “Coach” OR “Navigator”) AND (“trial” OR “controlled trial” OR “experimental study” OR “quasi-experimental study”).
Study Selection
All included studies examined PSIs as the main intervention, in which the trained peers were not healthcare professionals and were cancer survivors who had received treatment, laypersons in similar age groups, or intimate partners or family members of the patient. The PSIs took place as one-on-one interactions, and the effects were measured on the patients with cancer who received the intervention. A broad definition of PSI was used, so that studies of all interventions designed to help patients with cancer improve their physical and psychosocial outcomes (e.g., QOL, healthy behavior, sexuality) were included. In all studies, peer assistance was given individually to the person with cancer, either via face-to-face or telephone contact. For studies that reported the outcomes of patients and peers (Bultz et al., 2000; Campbell et al., 2007), only patient data were used. When the peer-led intervention was provided to an experimental group and a control group, but the interventions had different intensities, the study was included (Pinto, Stein, & Dunsiger, 2015a, 2015b; Schover et al., 2011). Studies were excluded if they had the following characteristics:
• They used group dynamics or a group-based format of peers with cancer.
• The peer-led interventions were supplemental to direct intervention from a healthcare professional.
• The patients with cancer were receiving hospice or palliative care.
• The peer-led support was a self-help group (participants were included in a group of individuals with similar illness and received support from those group members) or was Internet-based.
All retrieved titles and abstracts were added to a reference management database and screened for duplicates. Titles and abstracts of articles were read to identify and exclude those articles not meeting the inclusion criteria. Then, articles appearing to meet the criteria were read in full by the research assistant and the current authors. During this independent reading of articles, the current authors determined the final selection of articles, and reference lists of these articles were reviewed to identify other potential articles. A standard data extraction form was used to screen the titles and abstracts of each article to ensure they met the eligibility criteria. An independent nurse methodologist resolved disagreements regarding eligibility and verified the studies that were ultimately selected. The institutional review board at Kyungpook National University in Daegu, South Korea, approved the study.
Data Extraction
The following data were extracted from each study:
• Author
• Year of publication
• Patient nationality
• Study design
• Type of cancer
• Sample size
• Control conditions
• PSI details (peer characteristics, training, and counseling experience; content of intervention; theoretical framework; intervention method; number of sessions; intervention period; duration of each session, and follow-up times)
The outcomes of the PSI were cancer symptoms, coping, emotional health, QOL, self-efficacy, sexuality, social support, and health-related behavior.
Risk of Bias
RCTs and non-RCTs were independently assessed for methodologic quality by the current authors using the risk of bias tool, developed by the Cochrane Bias Methods Group (Higgins & Green, 2011). Each study was evaluated according to four criteria: random sequence generation, allocation concealment, blinding of data collectors, and blinding of outcome assessors. Each criterion was judged to have a high or low risk of bias. If a study was determined to have a high risk of bias for any one criterion, then it was considered to have a high risk of bias overall (Violette et al., 2015). When evaluating the risk of bias was difficult (such as when no information was available concerning allocation concealment or the methods of blinding or randomization, or when the information was insufficiently detailed), the current authors judged the risk of bias to be uncertain.
Statistical Analysis
The treatment and control groups were compared by calculation of standardized mean differences (SMDs) and 95% confidence intervals (CIs). Means and standard deviations, frequencies, and percentages before and after the interventions were used to calculate SMDs (Cohen’s d) (Becker, 1988). All ESs reported in this study were calculated using Cohen’s d. A Cohen’s d of 0–0.3 indicates a small ES, 0.3–0.6 indicates a moderate ES, and greater than 0.6 indicates a large ES (Cohen, 1988). Each ES was weighted by the inverse of its variance for calculating the SMD. This approach gives more weight to studies with larger sample sizes and reduces the imprecision of the pooled-effect estimate (Higgins & Green, 2011). The results across studies were pooled using the DerSimonian-Laird random effects model, in which tau was estimated by the method of moments (DerSimonian & Laird, 1986). I2 was used to describe heterogeneity among studies, and its value identified studies as having low (0–0.25), moderate (0.26–0.75), or high heterogeneity (0.76–1) (Higgins, Thompson, Deeks, & Altman, 2003). The current authors assumed real differences among the studies as well as sampling errors and, therefore, conservatively used the random effects model. A meta-analysis for a particular outcome was conducted when data were available from at least two studies. Subgroup analyses were used for separate analyses of studies according to the following characteristics:
• Study design
• Counseling experience of peers
• Application of a theoretical framework
• Role of healthcare professionals during the peer intervention
• Presence of a certain supportive action (such as counseling) by a healthcare professional for peers who possibly experience trauma symptoms or burnout
• Type of peers (i.e., intimate partners or cancer survivors)
• Control conditions
• Risk of bias
• Methods of communicating between the peer groups and the recipients
Two studies did not report statistics of the experimental and control groups, and the current authors were unable to retrieve these data, even after contacting the author (Schover et al., 2006, 2011). These two studies were excluded.
A funnel plot of precision versus SMD was used to assess the potential of publication bias. The absence of bias yields a plot resembling a symmetrical funnel; the presence of bias, which could occur if studies with small sample sizes and no statistically significant effects were not published, yields an asymmetrical funnel with a gap in a bottom corner (Higgins & Green, 2011). Egger’s regression test was complementarily used to determine the publication bias (Egger, Davey Smith, Schneider, & Minder, 1997). Meta-analysis was conducted using Comprehensive Meta-Analysis software, version 3.0. A p value of less than 0.05 was considered to be statistically significant, and all statistical tests were two-sided.
Findings
Literature Search and General Characteristics
The current authors initially identified 8,977 reports from the six databases. After screening titles, abstracts, and full texts, the current authors selected 16 RCTs and 2 non-RCTs for inclusion (see Figure 1). Tables 1 and 2 describe the characteristics of these 18 studies. Fifteen studies were conducted in North America (Canada and the United States), 2 in South Korea, and 1 in Australia. Ten studies were published within the past 10 years, the mean age of study participants ranged from 45–63 years, the sample size ranged from 30–367 participants, and the total number of patients was 2,254. Most studies examined patients with breast cancer (n = 12), followed by prostate cancer (n = 4), gastrointestinal cancer (n = 1), and multiple cancers (n = 1). The interventions were implemented at the time of diagnosis, after surgery, after surgery and ongoing adjuvant treatment, after primary treatment, or after cancer recurrence. 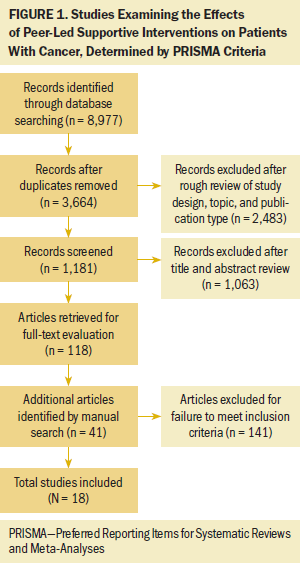
Risk of Bias
The risk of bias, assessed using Cochrane criteria, indicated that 15 studies had a high risk of bias and three had a low risk of bias (see Table 3). All 16 RCTs adequately randomized the enrolled patients. Six trials were classified as having used adequate concealment, 13 trials as having used adequate blinding of data collectors, and 10 trials as having used adequate blinding of the outcome assessor. No trial reported whether the data analysts were blinded. 
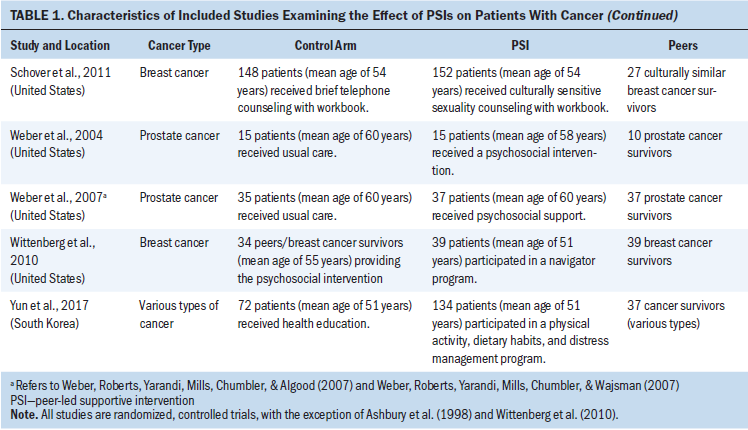
Description of Intervention and Control Conditions
The PSIs were designed to provide psychosocial support (Ashbury, Cameron, Mercer, Fitch, & Nielsen, 1998; Bultz et al., 2000; Campbell et al., 2007; Crane-Okada et al., 2012; Giese-Davis et al., 2016; Lee et al., 2013; Napoles et al., 2015; Porter et al., 2009; Samarel, Fawcett, & Tulman, 1997; Weber et al., 2004; Weber, Roberts, Yarandi, Mills, Chumbler, & Algood, 2007; Weber, Roberts, Yarandi, Mills, Chumbler, & Wajsman, 2007; Wittenberg et al., 2010), sexuality support (Chambers et al., 2015; Schover et al., 2006, 2011), health behavior support (Pinto et al., 2015a, 2015b; Yun et al., 2017), and QOL support (Gotay et al., 2007). The types of peers used for the PSIs were cancer survivors, individuals of a similar age, spouses, intimate partners, family members, and friends. All interventions were conducted by trained peers. Thirteen studies reported the training time for peers (range = 2–48 hours) or the number of training sessions for peers (range = 3–8 sessions). The peers’ ages were reported in 10 studies (range = 53–68 years, mean = 59 years). Six studies reported that the peers had counseling experience. Thirteen studies reported that healthcare professionals had roles in monitoring, supervising, educating, facilitating, ensuring quality control, or mentoring in regard to the peers’ interventions. 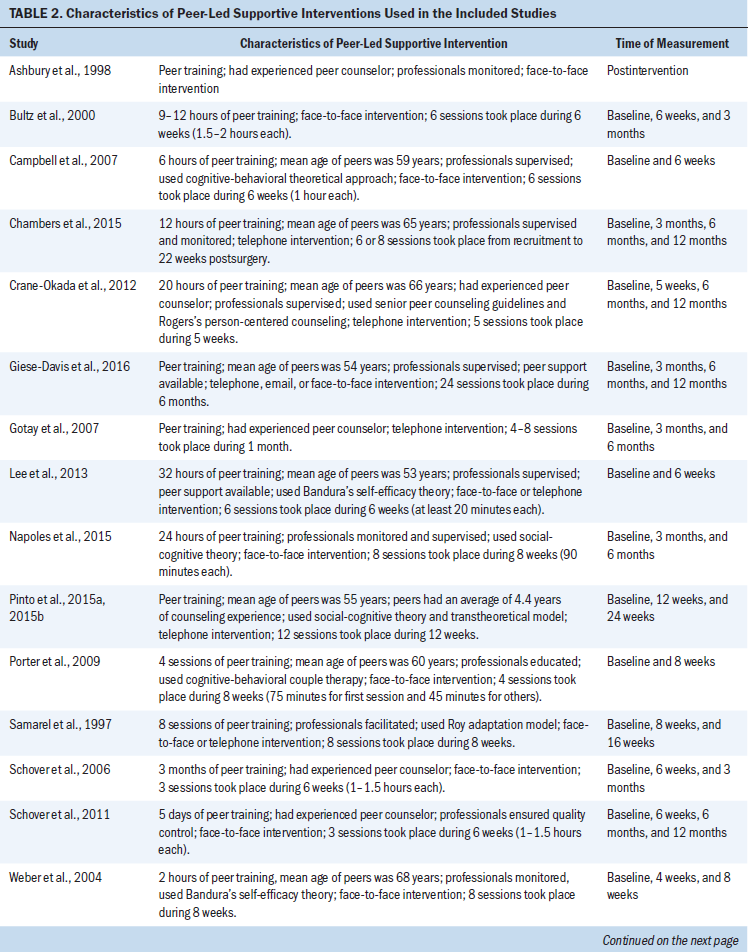

The PSIs varied greatly in terms of the number of sessions and their timing. In particular, the number of sessions ranged from 3–24 (mean = 8.8), and the duration of sessions ranged from 4–24 weeks (mean = 10.9 weeks). Ten studies reported the length of each session, which ranged from 20 minutes to 2 hours; in some cases, the participants determined the length of each session. The PSIs occurred as face-to-face interactions in nine studies, as telephone calls in five studies, through a combination of face-to-face interactions and telephone calls in two studies, and through a combination of face-to-face interactions, telephone calls, and email messages in two studies. A theoretical framework (cognitive-behavioral theory, Rogers’s person-centered counseling, Bandura’s self-efficacy theory, social-cognitive theory, the transtheoretical model, Roy adaptation model, leadership model) was used for the interventions in 10 studies. Seventeen of the 18 studies used more than two follow-up assessments. Members of the control groups received attentional control (Chambers et al., 2015; Pinto et al., 2015a, 2015b; Porter et al., 2009; Samarel et al., 1997; Schover et al., 2011; Wittenberg et al., 2010; Yun et al., 2017), no intervention (Ashbury et al., 1998; Crane-Okada et al., 2012), usual care following assignment to a waiting list (Bultz et al., 2000; Napoles et al., 2015; Schover et al., 2006), or usual care (Campbell et al., 2007; Chambers et al., 2015; Giese-Davis et al., 2016; Gotay et al., 2007; Lee et al., 2013; Weber et al., 2004; Weber, Roberts, Yarandi, Mills, Chumbler, & Algood, 2007; Weber, Roberts, Yarandi, Mills, Chumbler, & Wajsman, 2007).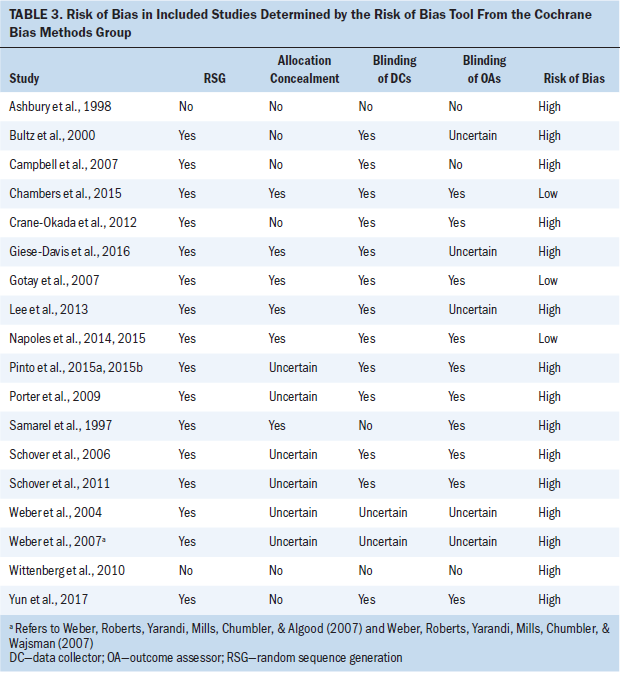
Patient Outcomes
The included studies used diverse instruments to measure patient outcomes. Five studies evaluated cancer symptoms by recording fatigue, bowel symptoms, hormonal symptoms, urinary symptoms, breast cancer–specific symptoms, symptom distress, appetite loss, constipation, diarrhea, dyspnea, insomnia, nausea, vomiting, and pain. Seven studies evaluated coping by measuring cognitive avoidance, fatalism, “fighting spirit,” helplessness, hopelessness, impact of cancer, post-traumatic growth, coping responses, cancer-specific trauma symptoms, and intrusive thoughts. Eleven studies evaluated emotional health by measuring anger-hostility, anxious preoccupation, confusion-bewilderment, depression-dejection, tension-anxiety, vigor-activity, anxiety, depression, psychosocial distress, somatization, blaming others, and blaming oneself. Two studies evaluated health-related behaviors by examining dietary habits, physical activity, and adherence to medication or treatment. Ten studies evaluated QOL using various tools: the Functional Living Index–Cancer, Functional Assessment of Cancer Therapy, Expanded Prostate Cancer Index Composite, physical function and mental health scales of the SF-36®, Inventory of Functional Status–Cancer, Ryff Happiness Scale, Quality of Life Index, UCLA Prostate Cancer Index, and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire. These tools were employed to measure functional well-being (functional status, physical function, bowel function, hormonal function, urinary function, breast cancer–specific function, social function, social activity, role function, cognitive function); overall well-being (global health status, overall QOL, enjoyment of life, family satisfaction, socioeconomic satisfaction, family satisfaction, physical well-being, emotional well-being, social and family well-being, breast cancer–specific well-being); and relationship quality (relationship with doctor, desire to see and be with family and friends, quality of interpersonal relationships). Five studies evaluated self-efficacy based on activity efficacy, coping efficacy, symptom management efficacy, self-efficacy regarding cancer, self-efficacy for self-management, and emotional self-efficacy. Seven studies evaluated sexuality based on measurement of marital satisfaction, sexual function, intimacy with the spouse, masculineself-esteem, sexual needs, sexual self-confidence, marital interaction, and quality of relationship with spouse. Six studies examined social support outcomes based on functional social support and desire to learn from cancer resources.
Effect of Peer-Led Supportive Interventions on Patient Outcomes
Table 4 shows the combined ES (SMD) of each trial and the ES of each trial regarding cancer symptoms (n = 6), coping (n = 7), emotional health (n = 11), QOL (n = 10), self-efficacy (n = 5), sexuality (n = 7), social support (n = 6), and health-related behaviors (n = 2). The ESs of the 16 studies ranged from –0.11 (95% CI [–0.41, 0.19]) for Chambers et al. (2015) to 0.89 (95% CI [0.45, 1.34]) for Wittenberg et al. (2010). The weighted average (w) indicated a small ES (wES = 0.2; 95% CI [0.12, 0.29]; p < 0.001). The heterogeneity was significant and moderate (p < 0.001, Q [15] = 31.7, I2 = 53%). 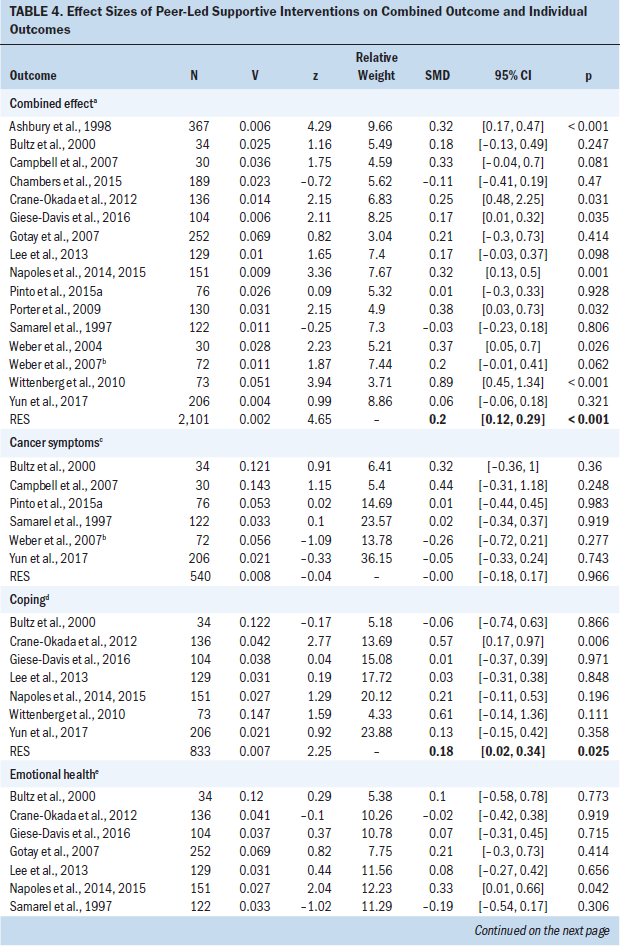
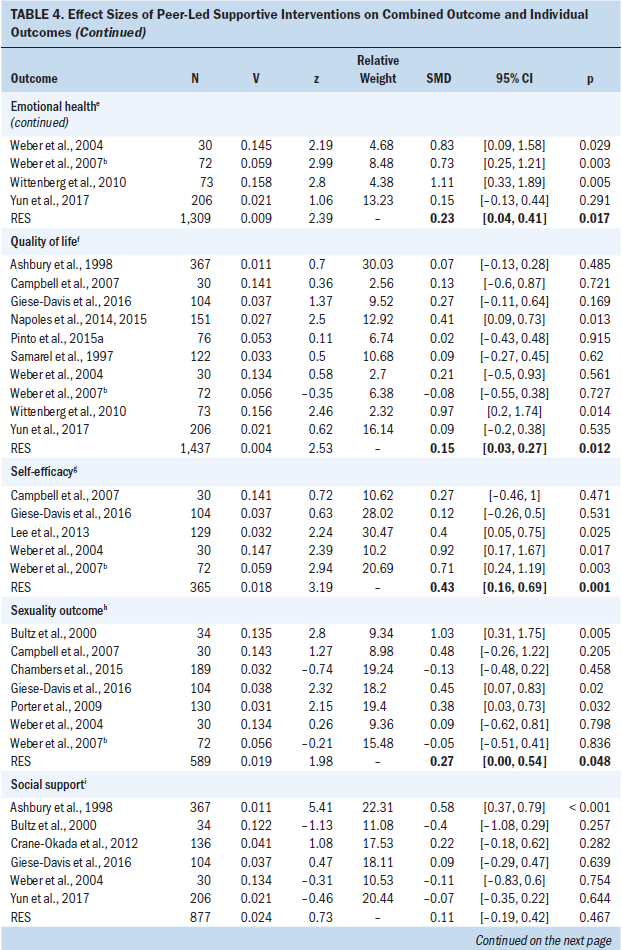
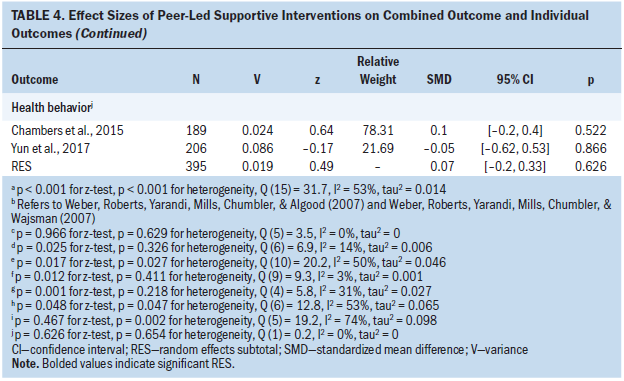
Analysis of the six studies that measured cancer symptoms indicated no significant heterogeneity (p = 0.629, Q [5] = 3.5, I2 = 0%); pooling of these six studies indicated that the PSI group had symptoms similar to the control group (wES = 0.00; 95% CI [–0.18, 0.17]; p = 0.966). Analysis of the seven studies that measured coping indicated that the PSI group had a small improvement relative to the control group (wES = 0.18; 95% CI [0.02, 0.34]; p = 0.025); no significant heterogeneity was observed among these studies (p = 0.326, Q [6] = 6.9, I2 = 14%). Analysis of the 11 studies that measured emotional health indicated that the PSI group had a small improvement relative to the control group (wES = 0.23; 95% CI [0.04, 0.41]; p = 0.017); the heterogeneity among these studies was moderate and significant (p = 0.027, Q [10] = 20.2, I2 = 50%). Analysis of the 10 studies that measured QOL indicated that the PSI group had a small improvement relative to the control group (wES = 0.15; 95% CI [0.03, 0.27]; p = 0.012); no significant heterogeneity was observed among these studies (p = 0.411, Q [9] = 9.3, I2 = 3%). Analysis of the five studies that measured self-efficacy indicated that the PSI group had a moderate improvement relative to the control group (wES = 0.43; 95% CI [0.16, 0.69]; p = 0.001); no significant heterogeneity was observed among these studies (p = 0.218, Q [4] = 5.8, I2 = 31%). Analysis of the seven studies that measured sexuality indicated that the PSI group had a small improvement relative to the control group (wES = 0.27; 95% CI [0.00, 0.54]; p = 0.048); the heterogeneity among these studies was significant and moderate (p = 0.047, Q [6] = 12.8, I2 = 53%). Analysis of the six studies that measured social support indicated that the PSI group had no significant improvement relative to the control group (wES = 0.11; 95% CI [–0.19, 0.42]; p = 0.467); the heterogeneity among these studies was significant and moderate (p = 0.002, Q [5] = 19.2, I2 = 74%). Analysis of the two studies that measured healthy behavior indicated that the PSI group and the control group had similar outcomes (wES = 0.07; 95% CI [–0.2, 0.33]; p = 0.626); no significant heterogeneity was observed between these studies (p = 0.654, Q [1] = 0.2, I2 = 0%).
Subgroup Analyses
The current authors also performed subgroup analyses of studies that did or did not apply a theoretical framework, used different control conditions, used different study designs, used peers with or without counseling experience, had roles for healthcare professionals during the intervention, did or did not provide supportive action for peers who experienced trauma symptoms, had different types of peers (spouse versus cancer survivor), and had different levels of bias (see Table 5). The results indicate that PSIs had a significant effect on coping when they employed a theory-based intervention (wES = 0.21; 95% CI [0.01, 0.42]; p = 0.037). PSIs also had a significant effect on emotional health when the control group received usual care or no intervention (wES = 0.24; 95% CI [0.05, 0.43]; p = 0.012), when the study had a low risk of bias (wES = 0.3; 95% CI [0.03, 0.57]; p = 0.031), and when the PSI was delivered through face-to-face interactions (wES = 0.47; 95% CI [0.18, 0.75]; p = 0.001). PSIs had a significant effect on QOL in the RCTs (wES = 0.16; 95% CI [0.02, 0.3]; p = 0.026), when the control group received usual care or an intervention (wES = 0.16; 95% CI [0.02, 0.3]; p = 0.028), when peers were cancer survivors (wES = 0.17; 95% CI [0.02, 0.32]; p = 0.025), and when the PSI was delivered through face-to-face interactions (wES = 0.66; 95% CI [0.3, 1.01]; p < 0.001). PSIs had a significant effect on self-efficacy when a theory-based intervention was applied (wES = 0.53; 95% CI [0.28, 0.77]; p < 0.001). PSIs had a significant effect on sexuality when the peers were spouses or intimate partners (wES = 0.53; 95% CI [0.18, 0.89]; p = 0.003) and when the PSI was delivered through face-to-face interactions (wES = 0.34; 95% CI [0.01, 0.67]; p = 0.042). PSIs also had a significant effect on social support when the peers had counseling experience (wES = 0.44; 95% CI [0.1, 0.78]; p = 0.012). 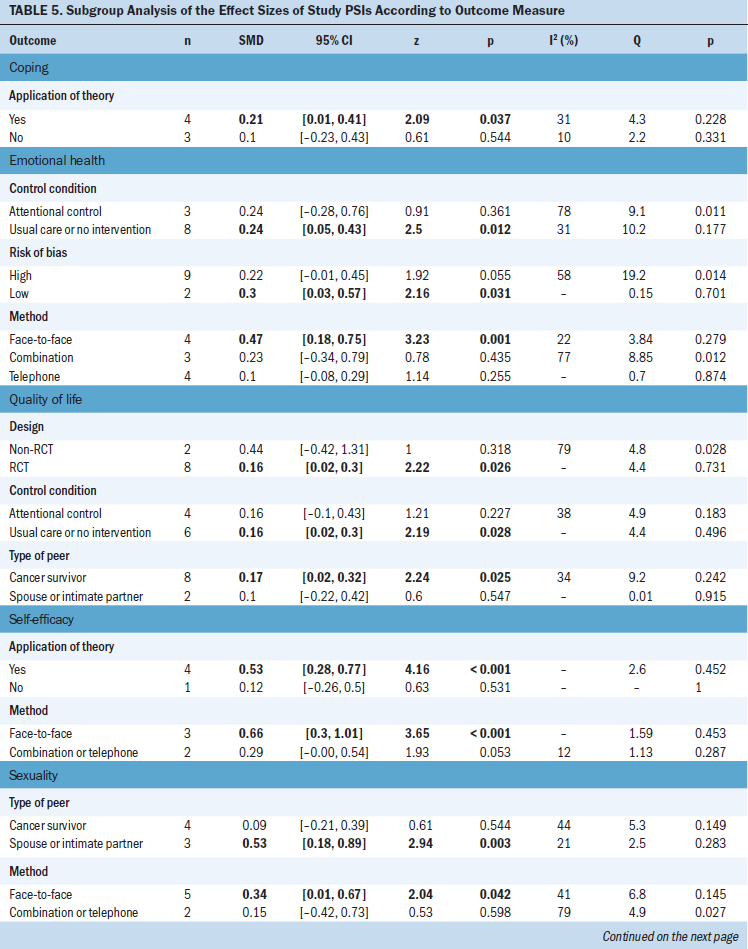
Publication Bias
A funnel plot of all 16 studies was symmetric (studies with high precision are plotted near the average, and studies with low precision are spread evenly on both sides of the average), indicating no evidence of potential publication bias. The results of Egger’s regression test support this conclusion (bias = 0.94, t = 1.28, df = 14, p = 0.223).
Discussion
The purpose of this meta-analysis was to provide an initial assessment of the effect of PSIs on the outcomes of patients with cancer. Overall, PSIs appeared to provide small benefits (ES = 0.2). More specifically, PSIs had moderate effectiveness in improving self-efficacy and small effectiveness in improving sexuality, emotional health, coping, and QOL. Previous reviews suggested that peer support had a positive effect on psychological adaptation, either directly (by decreasing feelings of isolation, encouraging healthy behaviors, and promoting positive psychological states) (Macvean et al., 2008; Newell, Sanson-Fisher, & Savolainen, 2002) or indirectly (by buffering the impact of stress on health, reframing threat appraisals, and improving coping responses and behaviors) (Hoey et al., 2008).
The current authors can suggest several reasons why the PSIs had small ESs on sexuality, emotional health, coping, and QOL. No RCTs screened for participants’ willingness to receive support. The trials examined in the current study mostly recruited patients through hospitals, and eligibility was based primarily on diagnosis, treatment status, or disease status. Patients with cancer who have low levels of psychological distress, who are not open to receiving support, or who have adequate social support at baseline are less likely to experience psychosocial improvements (Crane-Okada et al., 2012; Schover et al., 2011). A related reason for these small ESs is that the optimal intensity and timing of the PSI may differ among individuals (Crane-Okada et al., 2012). As treatment progresses, patients with cancer may learn from whom they need support, when they need support, and the type of support they need. The intensity and timing of the PSI should be tailored to a patient’s individual needs.
The current authors further analyzed the small ESs of the PSI by performing subgroup analyses. Subgroup analyses indicated that the effect of the PSI on sexuality, emotional health, coping, self-efficacy, QOL, and social support was stronger for studies with theoretical underpinnings, when the quality of the methodology was high (such as use of an RCT design and a low risk of bias), when the intervention was delivered through face-to-face interactions, or when the peers had counseling experience. Findings from the current study indicate that using a theoretical framework to guide the interventions led to greater effects in coping and self-efficacy. A previous meta-analysis of nurse-led supportive interventions for patients with cancer reported better outcomes when using approaches based on a theoretical framework (Suh & Lee, 2017). The use of an appropriate theoretical framework can help guide the design of health messages (Basil & Witte, 2012; O’Keefe, 2012), including the use of appropriate emotional appeals (Turner, 2012); consideration of differences in health literacy (Chambers, Ferguson, Gardiner, Aitken, & Occhipinti, 2013); and customization of messages for individuals with different stages of motivation (Noar & Van Stee, 2012). The tailoring of supportive care for participants using a theoretical framework improves different types of health outcomes (Albarracín et al., 2005; Cohen, Gottlieb, & Underwood, 2001; Hardeman et al., 2002).
Another reason for the small ESs may be that methodologic issues led to systematic bias and diminished the ESs and their statistical significance. The current authors’ subgroup analyses showed that PSIs had larger effects on emotional health and QOL in studies that were RCTs and that had low risk of bias. A previous meta-analysis of nurse-led supportive interventions for patients with cancer reported better outcomes in terms of emotional distress and QOL for studies that were RCTs (Suh & Lee, 2017). The methodologic limitations of a study may bias the results toward no effect or increased effect (Higgins, Altman, & Sterne, 2011). More rigorous studies, such as RCTs and those with a low risk of bias, are more likely to yield reliable results.
Use of different types of peers for the PSIs may influence specific outcomes. The effect of the PSI on QOL became stronger when the peers were cancer survivors (ES = 0.17) rather than spouses or intimate partners (not significant). However, the effect of the PSI on sexuality was stronger when the peers were spouses or intimate partners (ES = 0.53) rather than cancer survivors (ES = 0.27). Most people consider sexuality to be a private issue and may be unwilling to discuss this topic with individuals who are not spouses or intimate partners. The PSIs led by cancer survivors may have a positive effect on sexuality when the intervention is guided by educational materials, even though participants may be unwilling to discuss sexuality issues (Schover et al., 2006). The current authors’ subgroup analysis of the effect of PSIs on QOL indicated that the use of cancer survivors (rather than spouses or intimate partners) as peers may improve outcomes. One study tested the efficacy of telephone counseling by senior peers with no history of cancer for older women after breast cancer surgery; the patients in this study reported that the PSI would have been more helpful if the peer counselor had been a breast cancer survivor (Crane-Okada et al., 2012). Cancer is a life-altering event, and survivors have fears related to recurrence and face spiritual challenges related to having survived this illness. Cancer survivors may have a better understanding of this response and be better able to empathize and provide improvements in multiple dimensions of QOL, including physical and psychosocial functioning, cancer symptoms, and overall well-being (Ferrell, Dow, Leigh, Ly, & Gulasekaram, 1995).
A peer’s counseling experience may significantly affect perceived social support among patients with cancer, as suggested by the current authors’ subgroup analysis. The effects of PSIs on perceived social support was not significant in the pooled analysis; however, patients with cancer who received PSIs from peers with counseling experience showed moderate improvement in perceived social support (ES = 0.44). Given the potential vulnerability of patients with histories of cancer, recognizing the negative effect of their cancer-related distress is important (Giese-Davis et al., 2006; Söllner et al., 2001). A healthcare professional or experienced counselor may more easily identify these signs of distress, provide a safe and confidential place to talk, and help to turn a personal health crisis into a chance for hope and healing.
In addition, the effect of a PSI on emotional health, self-efficacy, and sexuality was stronger for studies with only face-to-face interactions, rather than only telephone calls or mixed types of interactions. In contrast, studies of patients with psychiatric diseases suggested that interventions using the Internet or telephone were as effective as face-to-face interventions in engaging the participants (Andersson, Cuijpers, Carlbring, Riper, & Hedman, 2014; Steele, Mummery, & Dwyer, 2007; Wagner, Horn, & Maercker, 2014). The effectiveness of a specific type of intervention depends on the medical condition of the patients. To increase the impact of an intervention, determining the delivery mode and other characteristics that are best suited to different target groups is important.
Despite the current authors’ subgroup analysis, this study found that the PSIs did not reduce cancer symptoms or alter health-related behaviors. Nurse-led supportive interventions (Suh & Lee, 2017) had a moderate effect on improving cancer symptoms (ES = 0.33) and a large effect on increasing healthy behaviors (self-care) (ES = 0.64). This indicates that the effects of nurses and peers in providing supportive interventions for patients with cancer are clearly distinguishable. In particular, nurses improve symptom management and health promotion, whereas peers improve self-efficacy (ES = 0.43). Empathetic sharing of experiences with patients who have cancer is generally beyond the scope of healthcare professionals. The success of peers may be explained by Bandura’s (1997) self-efficacy theory, which uses concepts like mastery and vicarious experience. The PSIs provided the opportunity to see others successfully manage problems related to cancer diagnosis and treatment. These findings support the use of PSIs as a supplemental component of healthcare professional–led cancer care.
The current authors’ meta-regression analysis showed that the ES was not affected by the number of sessions (p = 0.55), intervention period (p = 0.951), duration of the session (p = 0.273), or sample size (p = 0.86). This is consistent with a previous review that examined the effect of psychosocial interventions on patients with cancer (Okuyama, Jones, Ricklefs, & Tran, 2015; Suh & Lee, 2017). The number or duration of PSIs may not adequately represent the intensity or “dose” of the intervention, the opportunity for establishing a good relationship, the quality of the interaction, or a trust-based relationship where outcome effects may be more apparent. Consequently, different methods may be needed to measure the quality of PSIs.
Strengths and Limitations
The strengths of the current study include the use of a comprehensive search for publications that used a homogeneous peer-led supportive model (one-on-one interaction) for patients with cancer, assessment of study eligibility and data abstraction by independent researchers, appraisal of the risk of bias, and use of moderator analyses. The results presented in this article broaden understanding of the many variables that should be considered when designing supportive interventions for patients with cancer based on their psychosocial needs. This meta-analysis of the effects of peer-led supportive interventions on patients with cancer is the first analysis of these trials in 20 years. However, it has several limitations. The trials included were mostly of people with breast or prostate cancer, which are cancers with strong gender specificity, so the psychosocial needs of these groups skewed the study statistics; in fact, this simply reflects the reality that PSIs are more commonly evaluated for patients with breast or prostate cancer than with other cancer types. Because patients with breast or prostate cancer often have problems with sexuality, which most people consider to be a private issue, researchers may believe that a spouse or intimate partner would be more effective in delivering these interventions. In addition, patients with breast or prostate cancer had longer survival times than those with other cancer types, so these patients may be more interested in learning life skills that improve QOL after cancer diagnosis. They also may be in the midst of teachable moments and, therefore, be more likely to actively participate in the interventions.
Another limitation is that the mean age of the study participants ranged from 45–63 years. Considering that cancer can occur at any age, that a need exists to address a broad range of patients, and that more than half of all cancers occur in adults who are aged 65 years or older (White et al., 2014), this limits the generalizability of the results. Examination of the effect of a PSI in older adult patients with cancer is challenging (Gollhofer et al., 2015). In addition, for trials of supportive interventions involving peers, having adult patients with cancer participate voluntarily may be difficult because of a reduced ability to recognize their motivations, such as wanting to help others (Jenkins & Fallowfield, 2000) or wanting personal benefits (Catt, Langridge, Fallowfield, Talbot, & Jenkins, 2011). Despite the difficulties in studying older adult patients, further emphasis on social support for this population of patients with cancer may improve their physical and psychosocial functioning (Coll-Planas et al., 2017; Smith, Banting, Eime, O’Sullivan, & van Uffelen, 2017). Because older adult patients with cancer are better at self-managing their health than older adult patients with other chronic illnesses (Lee, 2016), peer-led supportive interventions based on the cancer care continuum, such as the Chronic Care Model (McCorkle et al., 2011), may be effective for older adult patients with cancer. Additional studies are needed to evaluate the effects of the peer-led care model on older adult patients with cancer in nursing practice.
The potential bias of the included trials must also be noted. The methodologic problems encountered, including high risk of bias, small sample sizes, and lack of long-term follow-ups, reduced the validity of the findings. The presence of so few trials with a low risk of bias examining PSIs means that the current body of knowledge about this topic is limited and that additional research is needed, as are improvements in the methodology of these future trials. In particular, future trials should clearly articulate the research methodology so that readers can better judge the risk of bias and more accurately interpret the findings. Well-designed RCTs, with adequate statistical power and appropriate outcome measures, must be conducted to confirm the current authors’ finding that PSIs provide a benefit for patients with cancer.
Implications for Nursing
A major responsibility of oncology nurses is to refer patients who are more vulnerable to distress and have a greater need for ongoing formal contact and support to valuable resources. The current review suggests that the PSI appears to be a valuable resource for patients with cancer. However, PSIs had only small effects on sexuality, emotional health, coping, and QOL and no significant effect on cancer symptoms and health-related behaviors. The current authors believe these shortcomings can be overcome by using a tiered evaluation that has a theoretical underpinning, a high-quality methodology, and an intervention that considers the intensity and timing of the PSI according to patients’ needs and characteristics, as well as by carefully selecting peers regarding their experience with interventions and according to patients’ specific needs or desired outcomes. Nurse-led supportive interventions and PSIs had different effects on different outcomes (Suh & Lee, 2017). Therefore, the combined use of two care models in caring for patients with cancer may benefit the healthcare team and patients who require multidimensional care. PSI programs in clinical settings require an infrastructure that ensures oversight of peers, which includes staff time for recruiting, supervising, and maintaining a pool of peers; patient referrals and matching; supervision and compliance with confidentiality of health information; and program evaluation. 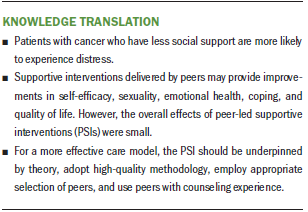
Conclusion
The findings of the current authors’ meta-analysis of the effect of PSIs on patients with cancer suggest that supportive interventions delivered by peers during cancer care appear to provide moderate improvements in self-efficacy and small improvements in sexuality, emotional health, coping, and QOL. Stronger outcomes were discovered in studies that were well-designed RCTs, had theoretical underpinnings, used appropriate selection of peers (cancer survivors versus spouse or intimate partner) according to the targeted outcome, and employed peers who had experience with counseling. The current review and meta-analysis further suggests that the combined use of the nurse-led care model and the peer-led care model in caring for patients with cancer may benefit the healthcare team and patients, who require comprehensive care to improve QOL.
About the Author(s)
Myung Kyung Lee, RN, PhD, OCN®, is an assistant professor and Soon-Rim Suh, RN, PhD, is a professor, both in the College of Nursing and Research Institute of Nursing Science at Kyungpook National University in Daegu, South Korea. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (NRF-2017R1A1A1A05001045). Both authors contributed to the conceptualization and design, completed the data collection, and contributed to the manuscript preparation. Lee provided the statistical support and the analysis. Suh can be reached at srsuh@knu.ac.kr, with copy to ONFEditor@ons.org. (Submitted July 2017. Accepted September 26, 2017.)
References
Albarracín, D., Gillette, J.C., Earl, A.N., Glasman, L.R., Durantini, M.R., & Ho, M.-H. (2005). A test of major assumptions about behavior change: A comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychological Bulletin, 131, 856–897. https://doi.org/10.1037/0033-2909.131.6.856
Andersson, G., Cuijpers, P., Carlbring, P., Riper, H., & Hedman, E. (2014). Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: A systematic review and meta-analysis. World Psychiatry, 13, 288–295. https://doi.org/10.1002/wps.20151
Andrykowski, M.A., Lykins, E., & Floyd, A. (2008). Psychological health in cancer survivors. Seminars in Oncology Nursing, 24, 193–201. https://doi.org/10.1016/j.soncn.2008.05.007
Ashbury, F.D., Cameron, C., Mercer, S.L., Fitch, M., & Nielsen, E. (1998). One-on-one peer support and quality of life for breast cancer patients. Patient Education and Counseling, 35, 89–100.
Bandura, A. (1997). Self-efficacy: The exercise of control. New York, NY: Macmillan.
Basil, M., & Witte, K. (2012). Health risk message design using the extended parallel process model. In H. Cho (Ed.), Health communication message design: Theory and practice (pp. 41–58). Thousand Oaks, CA: Sage.
Becker, B.J. (1988). Synthesizing standardized mean-change measures. British Journal of Mathematical and Statistical Psychology, 41, 257–278. https://doi.org/10.1111/j.2044-8317.1988.tb00901.x
Bultz, B.D., Speca, M., Brasher, P.M., Geggie, P.H., & Page, S.A. (2000). A randomized controlled trial of a brief psychoeducational support group for partners of early stage breast cancer patients. Psycho-Oncology, 9, 303–313.
Campbell, H.S., Phaneuf, M.R., & Deane, K. (2004). Cancer peer support programs—Do they work? Patient Education and Counseling, 55, 3–15. https://doi.org/10.1016/j.pec.2003.10.001
Campbell, L.C., Keefe, F.J., Scipio, C., McKee, D.C., Edwards, C.L., Herman, S.H., . . . Donatucci, C. (2007). Facilitating research participation and improving quality of life for African American prostate cancer survivors and their intimate partners: A pilot study of telephone-based coping skills training. Cancer, 109(Suppl. 2), 414–424. https://doi.org/10.1002/cncr.22355
Catt, S., Langridge, C., Fallowfield, L., Talbot, D.C., & Jenkins, V. (2011). Reasons given by patients for participating, or not, in phase 1 cancer trials. European Journal of Cancer, 47, 1490–1497. https://doi.org/10.1016/j.ejca.2011.02.020
Chambers, S.K., Ferguson, M., Gardiner, R.A., Aitken, J., & Occhipinti, S. (2013). Intervening to improve psychological outcomes for men with prostate cancer. Psycho-Oncology, 22, 1025–1034. https://doi.org/10.1002/pon.3095
Chambers, S.K., Occhipinti, S., Schover, L., Nielsen, L., Zajdlewicz, L., Clutton, S., . . . Dunn, J. (2015). A randomised controlled trial of a couples-based sexuality intervention for men with localised prostate cancer and their female partners. Psycho-Oncology, 24, 748–756. https://doi.org/10.1002/pon.3726
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates.
Cohen, S., Gottlieb, B.H., & Underwood, L.G. (2001). Social relationships and health: Challenges for measurement and intervention. Advances in Mind-Body Medicine, 17, 129–141.
Cohen, S., & Wills, T.A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98, 310–357.
Coll-Planas, L., del Valle Gómez, G., Bonilla, P., Masat, T., Puig, T., & Monteserin, R. (2017). Promoting social capital to alleviate loneliness and improve health among older people in Spain. Health and Social Care in the Community, 25, 145–157. https://doi.org/10.1111/hsc.12284
Cox, A.D. (1993). Befriending young mothers. British Journal of Psychiatry, 163, 6–18.
Crane-Okada, R., Freeman, E., Kiger, H., Ross, M., Elashoff, D., Deacon, L., & Giuliano, A.E. (2012). Senior peer counseling by telephone for psychosocial support after breast cancer surgery: Effects at six months. Oncology Nursing Forum, 39, 78–89. https://doi.org/10.1188/12.ONF.78-89
DerSimonian, R., & Laird, N. (1986). Meta-analysis in clinical trials. Controlled Clinical Trials, 7, 177–188.
Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315, 629–634.
Eng, E., & Young, R. (1992). Lay health advisors as community change agents. Family and Community Health, 15, 24–40.
Ferrell, B.R., Dow, K.H., Leigh, S., Ly, J., & Gulasekaram, P. (1995). Quality of life in long-term cancer survivors. Oncology Nursing Forum, 22, 915–922.
Giese-Davis, J., Bliss-Isberg, C., Carson, K., Star, P., Donaghy, J., Cordova, M.J., . . . Spiegel, D. (2006). The effect of peer counseling on quality of life following diagnosis of breast cancer: An observational study. Psycho-Oncology, 15, 1014–1022. https://doi.org/10.1002/pon.1037
Giese-Davis, J., Bliss-Isberg, C., Wittenberg, L., White, J., Star, P., Zhong, L., . . . Spiegel, D. (2016). Peer-counseling for women newly diagnosed with breast cancer: A randomized community/research collaboration trial. Cancer, 122, 2408–2417. https://doi.org/10.1002/cncr.30036
Gollhofer, S.M., Wiskemann, J., Schmidt, M.E., Klassen, O., Ulrich, C.M., Oelmann, J., . . . Steindorf, K. (2015). Factors influencing participation in a randomized controlled resistance exercise intervention study in breast cancer patients during radiotherapy. BMC Cancer, 15, 186. https://doi.org/10.1186/s12885-015-1213-1
Gotay, C.C., Moinpour, C.M., Unger, J.M., Jiang, C.S., Coleman, D., Martino, S., . . . Albain, K.S. (2007). Impact of a peer-delivered telephone intervention for women experiencing a breast cancer recurrence. Journal of Clinical Oncology, 25, 2093–2099. https://doi.org/10.1200/JCO.2006.07.4674
Hardeman, W., Johnston, M., Johnston, D., Bonetti, D., Wareham, N., & Kinmonth, A.L. (2002). Application of the theory of planned behaviour in behaviour change interventions: A systematic review. Psychology and Health, 17, 123–158. https://doi.org/10.1080/08870440290013644a
Higgins, J.P., Altman, D.G., & Sterne, J.A. (2011). Assessing risk of bias in included studies. In J.P.T. Higgins & S. Green (Eds.), Cochrane handbook for systematic reviews of interventions [v.5.1.0]. London, England: Cochrane Collaboration.
Higgins, J.P.T., & Green, S. (Eds.). (2011). Cochrane handbook for systematic reviews of interventions [v.5.1.0]. Retrieved from http://www.cochrane.org/handbook
Higgins, J.P., Thompson, S.G., Deeks, J.J., & Altman, D.G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327, 557–560. https://doi.org/10.1136/bmj.327.7414.557
Hodgkinson, K., Butow, P., Hobbs, K.M., & Wain, G. (2007). After cancer: The unmet supportive care needs of survivors and their partners. Journal of Psychosocial Oncology, 25, 89–104. https://doi.org/10.1300/J077v25n04_06
Hoey, L.M., Ieropoli, S.C., White, V.M., & Jefford, M. (2008). Systematic review of peer-support programs for people with cancer. Patient Education and Counseling, 70, 315–337. https://doi.org/10.1016/j.pec.2007.11.016
Jenkins, V., & Fallowfield, L. (2000). Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. British Journal of Cancer, 82, 1783–1788. https://doi.org/10.1054/bjoc.2000.1142
Lee, M.K. (2016). Disability and quality of life in community-dwelling elderly cancer survivors: Case-control study in the Korean population. European Journal of Oncology Nursing, 24, 22–28. https://doi.org/10.1016/j.ejon.2016.08.003
Lee, R., Lee, K.S., Oh, E.-G., & Kim, S.H. (2013). A randomized trial of dyadic peer support intervention for newly diagnosed breast cancer patients in Korea. Cancer Nursing, 36, E15–E22. https://doi.org/10.1097/NCC.0b013e3182642d7c
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P., . . . Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology, 62, e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Lorig, K.R., & Holman, H. (2003). Self-management education: History, definition, outcomes, and mechanisms. Annals of Behavioral Medicine, 26, 1–7.
Macvean, M.L., White, V.M., & Sanson-Fisher, R. (2008). One-to-one volunteer support programs for people with cancer: A review of the literature. Patient Education and Counseling, 70, 10–24. https://doi.org/10.1016/j.pec.2007.08.005
McCorkle, R., Ercolano, E., Lazenby, M., Schulman-Green, D., Schilling, L.S., Lorig, K., & Wagner, E.H. (2011). Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: A Cancer Journal for Clinicians, 61, 50–62. https://doi.org/10.3322/caac.20093
Napoles, A.M., Ortiz, C., Santoyo-Olsson, J., Stewart, A.L., Gregorich, S., Lee, H.E., . . . Luce, J. (2015). Nuevo Amanecer: Results of a randomized controlled trial of a community-based, peer-delivered stress management intervention to improve quality of life in Latinas with breast cancer. American Journal of Public Health, 105(Suppl. 3), e55–e63. https://doi.org/10.2105/ajph.2015.302598
Napoles, A.M., Santoyo-Olsson, J., Ortiz, C., Gregorich, S., Lee, H.E., Duron, Y., . . . Stewart, A.L. (2014). Randomized controlled trial of Nuevo Amanecer: A peer-delivered stress management intervention for Spanish-speaking Latinas with breast cancer. Clinical Trials, 11, 230–238. https://doi.org/10.1177/1740774514521906
Newell, S.A., Sanson-Fisher, R.W., & Savolainen, N.J. (2002). Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. Journal of the National Cancer Institute, 94, 558–584.
Noar, S.M., & Van Stee, S.K. (2012). Designing messages for individuals in different stages of change. In H. Cho (Ed.), Health communication message design: Theory and practice (pp. 209–229). Thousand Oaks, CA: Sage.
O’Keefe, D.J. (2012). From psychological theory to message design: Lessons from the story of gain-framed and loss-framed persuasive messages. In H. Cho (Ed.), Health communication message design: Theory and practice (pp. 3–20). Thousand Oaks, CA: Sage.
Okuyama, S., Jones, W., Ricklefs, C., & Tran, Z.V. (2015). Psychosocial telephone interventions for patients with cancer and survivors: A systematic review. Psycho-Oncology, 24, 857–870. https://doi.org/10.1002/pon.3704
Pinto, B., Stein, K., & Dunsiger, S. (2015a). Peer mentorship to promote physical activity among cancer survivors: Effects on quality of life. Psycho-Oncology, 24, 1295–1302. https://doi.org/10.1002/pon.3884
Pinto, B.M., Stein, K., & Dunsiger, S. (2015b). Peers promoting physical activity among breast cancer survivors: A randomized controlled trial. Health Psychology, 34, 463–472. https://doi.org/10.1037/hea0000120
Porter, L.S., Keefe, F.J., Baucom, D.H., Hurwitz, H., Moser, B., Patterson, E., & Kim, H.J. (2009). Partner-assisted emotional disclosure for patients with gastrointestinal cancer: Results from a randomized controlled trial. Cancer, 115(Suppl. 18), 4326–4338. https://doi.org/10.1002/cncr.24578
Samarel, N., Fawcett, J., & Tulman, L. (1997). Effect of support groups with coaching on adaptation to early stage breast cancer. Research in Nursing and Health, 20, 15–26.
Schover, L.R., Jenkins, R., Sui, D., Adams, J.H., Marion, M.S., & Jackson, K.E. (2006). Randomized trial of peer counseling on reproductive health in African American breast cancer survivors. Journal of Clinical Oncology, 24, 1620–1626. https://doi.org/10.1200/JCO.2005.04.7159
Schover, L.R., Rhodes, M.M., Baum, G., Adams, J.H., Jenkins, R., Lewis, P., & Jackson, K.E. (2011). Sisters Peer Counseling in Reproductive Issues After Treatment (SPIRIT): A peer counseling program to improve reproductive health among African American breast cancer survivors. Cancer, 117, 4983–4992. https://doi.org/10.1002/cncr.26139
Siegel, R.L., Miller, K.D., & Jemal, A. (2015). Cancer statistics, 2015. CA: A Cancer Journal for Clinicians, 65, 5–29. https://doi.org/10.3322/caac.21254
Smith, G.L., Banting, L., Eime, R., O’Sullivan, G., & van Uffelen, J.G.Z. (2017). The association between social support and physical activity in older adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 14, 56. https://doi.org/10.1186/s12966-017-0509-8
Söllner, W., DeVries, A., Steixner, E., Lukas, P., Sprinzl, G., Rumpold, G., & Maislinger, S. (2001). How successful are oncologists in identifying patient distress, perceived social support, and need for psychosocial counselling? British Journal of Cancer, 84, 179–185. https://doi.org/10.1054/bjoc.2000.1545
Steele, R.M., Mummery, W.K., & Dwyer, T. (2007). Examination of program exposure across intervention delivery modes: Face-to-face versus internet. International Journal of Behavioral Nutrition and Physical Activity, 4, 7. https://doi.org/10.1186/1479-5868-4-7
Suh, S.-R., & Lee, M.K. (2017). Effects of nurse-led telephone-based supportive interventions for patients with cancer: A meta-analysis [Online exclusive]. Oncology Nursing Forum, 44, E168–E184. https://doi.org/10.1188/17.ONF.E168-E184
Turner, M.M. (2012). Using emotional appeals in health messages. In H. Cho (Ed.), Health communication message design: Theory and practice (pp. 59–72). Thousand Oaks, CA: Sage.
Violette, P.D., Agoritsas, T., Alexander, P., Riikonen, J., Santti, H., Agarwal, A., . . . Tikkinen, K.A. (2015). Decision aids for localized prostate cancer treatment choice: Systematic review and meta-analysis. CA: A Cancer Journal for Clinicians, 65, 239–251. https://doi.org/10.3322/caac.21272
Wagner, B., Horn, A.B., & Maercker, A. (2014). Internet-based versus face-to-face cognitive-behavioral intervention for depression: A randomized controlled non-inferiority trial. Journal of Affective Disorders, 152, 113–121. https://doi.org/10.1016/j.jad.2013.06.032
Weber, B.A., Roberts, B.L., Resnick, M., Deimling, G., Zauszniewski, J.A., Musil, C., & Yarandi, H.N. (2004). The effect of dyadic intervention on self-efficacy, social support, and depression for men with prostate cancer. Psycho-Oncology, 13, 47–60. https://doi.org/10.1002/pon.718
Weber, B.A., Roberts, B.L., Yarandi, H., Mills, T.L., Chumbler, N.R., & Algood, C. (2007). Dyadic support and quality-of-life after radical prostatectomy. Journal of Men’s Health and Gender, 4, 156–164. https://doi.org/10.1016/j.jmhg.2007.03.004
Weber, B.A., Roberts, B.L., Yarandi, H., Mills, T.L., Chumbler, N.R., & Wajsman, Z. (2007). The impact of dyadic social support on self-efficacy and depression after radical prostatectomy. Journal of Aging and Health, 19, 630–645. https://doi.org/10.1177/0898264307300979
White, M.C., Holman, D.M., Boehm, J.E., Peipins, L.A., Grossman, M., & Henley, S.J. (2014). Age and cancer risk: A potentially modifiable relationship. American Journal of Preventive Medicine, 46(Suppl. 1), S7–S15. https://doi.org/10.1016/j.amepre.2013.10.029
Wittenberg, L., Yutsis, M., Taylor, S., Giese-Davis, J., Bliss-Isberg, C., Star, P., & Spiegel, D. (2010). Marital status predicts change in distress and well-being in women newly diagnosed with breast cancer and their peer counselors. Breast Journal, 16, 481–489. https://doi.org/10.1111/j.1524-4741.2010.00964.x
Yun, Y.H., Kim, Y.A., Lee, M.K., Sim, J.A., Nam, B.-H., Kim, S., . . . Park, S. (2017). A randomized controlled trial of physical activity, dietary habit, and distress management with the Leadership and Coaching for Health (LEACH) program for disease-free cancer survivors. BMC Cancer, 17, 298.




