The Relationship Between Body Mass Index and Sexual Function in Endometrial Cancer
Objectives: To explore the association between pretreatment body mass index (BMI) and post-treatment sexual function in women treated for endometrial cancer.
Sample & Setting: 28 postmenopausal women treated with vaginal brachytherapy (VBT) took part in this multisite exploratory secondary analysis at the University of Pennsylvania and Northwestern University.
Methods & Variables: Secondary data analysis was used to determine if pretreatment BMI is associated with post-VBT sexual function in postmenopausal women treated for endometrial cancer at baseline and at six months post-treatment. Because of small sample size, participants were dichotomized according to enrollment BMI: 30 or greater (obese) and less than 30 (non-obese). The Female Sexual Function Index was used to assess sexual function, with total scores of 26.55 or less indicating sexual dysfunction.
Results: Both groups had poor sexual function at baseline. Although improved function was observed with time, neither group reached a score indicating healthy sexual function.
Implications for Nursing: Understanding factors that influence sexual health in patients with gynecologic cancer can improve post-treatment quality of life.
Jump to a section
Endometrial cancer is the most prevalent gynecologic cancer in the United States, with most cases occurring in women aged 50 years or older (Sorosky, 2012). The number of women diagnosed with endometrial cancer is expected to rise as the incidence of obesity, one of the most common risk factors, increases worldwide (Fader, Arriba, Frasure, & von Gruenigen, 2009). Obesity is a significant risk factor for endometrial cancer. In women who had never used postmenopausal hormone replacement therapy, a body mass index (BMI) of 35 or greater increased the risk for developing endometrial cancer by 441%, compared to a 51% decrease in risk in a woman with a BMI of less than 22.5 (McCullough et al., 2008). In addition, research indicates an association between obesity (BMI of 30 or greater) and lower recurrence-free survival, as well as lower overall survival rates, among patients with endometrial cancer (Ko et al., 2014).
One of the hallmark signs of endometrial cancer is abnormal postmenopausal bleeding; it affects about 90% of women with endometrial cancer, and its visible manifestation often prompts women to seek care and allows for detection of endometrial cancer at earlier stages of the disease (American Cancer Society [ACS], 2016a). Seventy-two percent of endometrial cancers are detected at stage I, whereas 3% are detected at stage III (Sorosky, 2012). According to an analysis from the National Cancer Database, vaginal brachytherapy (VBT) is the most common adjuvant treatment for early-stage endometrial cancer, and its use has been increasing (Rydzewski et al., 2016). VBT is a form of radiation therapy that involves the insertion of a cylindrical applicator containing radioactive material into the vagina at varying lengths, durations, and radiation doses, depending on the cancer stage (ACS, 2016b; Small et al., 2012).
All therapies associated with treatment of endometrial cancer (surgery, radiation therapy, and/or chemotherapy) have been shown to interfere, to some extent, with different aspects of sexuality, including desire, frequency, and satisfaction (Bergmark, Åvall-Lundqvist, Dickman, Henningsohn, & Steineck, 1999; Bruner, Jones, Buchanan, & Russo, 2006; Ganz, Rowland, Desmond, Meyerowitz, & Wyatt, 1998). However, past studies did not control for BMI; as a result, the independent contribution of BMI to post-VBT sexual function is not fully understood. Although VBT allows for more direct radiation administration to the area at highest risk and less radiation to other normal pelvic organs compared to traditional external beam radiation therapy, it is not without side effects that affect patients’ quality of life post-treatment. Research has noted the long-term negative consequences of VBT on the female reproductive system, including, but not limited to, vaginal stenosis, decreased vaginal lubrication, and vaginal atrophy, resulting in dyspareunia (ACS, 2016b).
Sexuality is a complex phenomenon that may be influenced by multiple factors, including chronic disease and its treatment. The World Health Organization (n.d.) defines sexual health as “a state of physical, emotional, mental and social well-being in relation to sexuality; it is not merely the absence of disease, dysfunction or infirmity” (para. 5). Studies comparing healthy obese women and non-obese women matched for age and menopausal status have shown that poor sexual functioning, reflected by a low score on the Female Sexual Function Index (FSFI), strongly correlates with BMI and that obesity negatively affects several aspects of sexuality (Esposito et al., 2007; Kolotkin, Zunker, & Østbye, 2012; Shah, 2009). The literature includes few conclusive studies examining the relationship between BMI and sexual function among patients treated for endometrial cancer (Damast et al., 2012, 2014). Investigation of the relationship between BMI and sexual function and the influence of these on quality of life can provide data that nurses can use to identify patients with endometrial cancer at risk for negative sexual health outcomes and allow for intervention and patient education. The aims of this study are to explore pretreatment BMI and its association with sexual function in women treated for endometrial cancer with VBT at baseline and at six months post-treatment, and to assess differences in change scores between obese and non-obese patients.
Methods
This study is a descriptive secondary analysis of data from the parent clinical trial (Feasibility Study of Adherence Intervention for Dilator Use Following Endometrial Cancer, R21CA140766), which was a feasibility study that assessed an intervention to increase adherence to vaginal dilator use to maintain vaginal patency after treatment for gynecologic cancer. This multisite study took place at the Abramson Cancer Center of the University of Pennsylvania in Philadelphia and at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago, Illinois. Institutional review board approval was obtained from the University of Pennsylvania, Northwestern University, and Emory University.
The inclusion and exclusion criteria were based on the parent study. Inclusion criteria were as follows:
• Aged 18 years or older
• Diagnosed with stage I–III endometrial cancer
• Had undergone a hysterectomy and oophorectomy with or without lymphadenectomy
• Had planned postoperative VBT at time of enrollment
• Had no history of prior pelvic radiation therapy or chemotherapy
Patients enrolled may have been sexually active or inactive before or during the study. Exclusion criteria were as follows:
• Diagnosed with stage IV endometrial cancer
• Did not speak or read English
• Had a mental or physical disability that, in the opinion of the physician, would preclude vaginal dilator use or instruction
A convenience sample of 42 participants with endometrial cancer were consented and randomized in the parent study; 28 participants completed the study, and 14 participants were lost to follow-up. This study analyzed the data from the 28 participants who completed the parent study. All participants received postoperative VBT consisting of fractionated high-dose-rate treatment based on the American Brachytherapy Society recommendations (Small et al., 2012).
The data from the parent study were obtained from patients’ medical records and in-person participant interviews. Data collected for this secondary analysis included patient demographics (age, race, marital status, education level), BMI, and FSFI scores. Participants’ height and weight were obtained at baseline, and BMI was calculated using the nonmetric formula: BMI = (weight [lb] divided by height2 [in.²]) x 703. According to the National Institutes of Health’s recommended guidelines, normal BMI is 18.5–24.9, overweight BMI is 25–29.9, and obese BMI is 30 or greater (Must et al., 1999). The FSFI is a brief 19-item multidimensional self-report scale that has demonstrated acceptable reliability (Cronbach alpha of 0.94), convergent and discriminant validity (p ≤ 0.001), and identification of diagnostic cutoff scores for potential classification of sexual dysfunction in female patients with cancer (Baser, Li, & Carter, 2012; Rosen et al., 2000). In addition, the FSFI has been shown to positively correlate with quality-of-life measures and has the ability to differentiate between women who have received different treatment methods (e.g., chemotherapy, radiation therapy), allowing for its usefulness in assessing sexual health in female cancer survivors (Baser et al., 2012). The FSFI evaluates sexual function across six domains (desire, arousal, lubrication, orgasm, satisfaction, and pain), and it assesses sexual function using a Likert-type scale with scores for each domain ranging from 0 (no sexual activity or did not attempt sexual intercourse) to 5 (high functioning within the specific domain); a total score that is equal to or less than 26.55 indicates possible impaired sexual function (Baser et al., 2012). In this study, the FSFI was completed at baseline (following surgery but pre-VBT) and at six months post-VBT.
All data were analyzed using IBM SPSS Statistics, version 21.0. Descriptive statistics were employed to describe the sample. FSFI scores for each group were analyzed using a paired samples t test and an independent sample t test between the two study groups. In addition, analysis of covariance (ANCOVA) was used to test any differences in mean FSFI scores at six months when controlling for baseline scores.
Results
Data from patients who completed the parent study were analyzed (N = 28). Mean age at enrollment was 57.9 years (SD = 10.7), and the range was 38–84 years. The mean pretreatment BMI was 30.3 (SD = 7.82), with a range of 17.4–50.6. Demographic data of the study population is shown in Table 1.
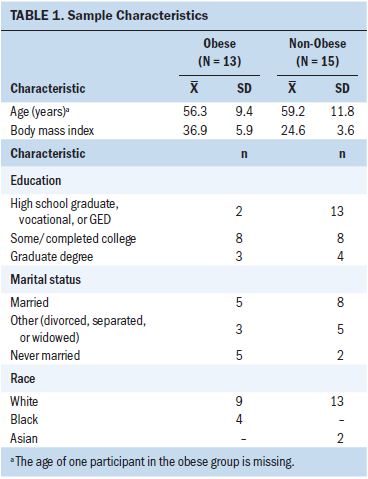
The study sample was divided into two groups according to enrollment BMI: (a) BMI of 30 or greater, grouped as obese (N = 13) and BMI of less than 30, grouped as non-obese (N = 15) (see Table 2). The mean baseline FSFI score for the non-obese group was 9.07 (SD = 8.55) and 13.53 (SD = 9.43) for the obese group. At baseline, 14 participants in the non-obese group and 12 participants in the obese group had an FSFI score of 26.55 or less. There were two outliers in the non-obese group for baseline FSFI score.
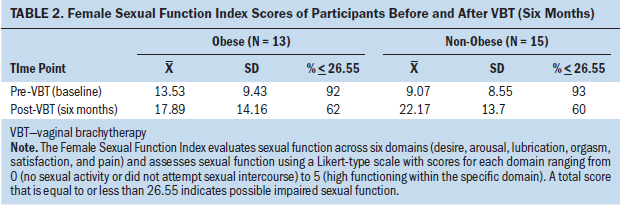
The mean FSFI score at six months for the non-obese group was 22.17 (SD = 13.7) and 17.89 (SD = 14.16) for the obese group. At six months, nine participants in the non-obese group and eight participants in the obese group had an FSFI score of 26.55 or less.
Neither the independent t test of mean FSFI scores between obese and non-obese participants at baseline nor the independent t test at six months were significant (t = –1.31, df = 26, p = 0.2 and t = 0.81, df = 26, p = 0.42, respectively). An independent t test of the change in mean FSFI scores between groups from baseline to six months was not significant (t = 1.87, df = 26, p = 0.07). However, a paired samples t test of the change in mean FSFI score between time points in the non-obese group was highly significant (t = 4.39, df = 14, p = 0.001, Cohen’s d = 1.13). A paired samples t test for the change in mean FSFI score between baseline and six months within the obese group was not significant (t = 1.187, df = 12, p = 0.26, Cohen’s d = 0.33). The ANCOVA for mean FSFI score at six months after adjusting for baseline FSFI score was also not significant (F = 2.49, df1 = 1, df2 = 25, p = 0.13).
Discussion
At baseline, participants in the non-obese group had more sexual dysfunction based on mean FSFI score than the obese group. However, this secondary analysis had important limitations. The baseline mean FSFI score for the non-obese group included the presence of two outliers, which may have influenced the spread of the data for that group. At baseline and at six months, no significant difference in mean FSFI score was noted between obese and non-obese participants. Both groups in this study had an increase in mean FSFI scores (indicating improved sexual function from baseline to six months), but neither group achieved healthy sexual function, as determined by a mean FSFI score of greater than 26.55. On average, the non-obese participants increased their mean FSFI score by more than 13 points from baseline to six months, and the obese participants increased their score by 4.4 points from baseline to six months. In this small exploratory sample, the change in mean FSFI score from baseline to six months was significant only for the non-obese group, not for the obese group. In addition, the effect size for improvement in post-VBT sexual function for the non-obese group was large (Cohen’s d = 1.13) compared to the small effect size of the obese group (Cohen’s d = 0.33). However, analysis of mean FSFI score at six months, after adjusting for baseline mean FSFI score, indicated that no significant differences were noted in mean FSFI score between the two groups at either time point.
Overall, pretreatment BMI was not associated with sexual function in this study group. However, the non-obese participants significantly increased their mean FSFI score from baseline to six months, which was not seen in the obese group. In addition, the changes seen in the non-obese group were more than two times greater than the changes seen in the obese group. Although the data trended toward significance and an improvement in sexual function (as determined by mean FSFI score) was observed within BMI groups, the primary study was not powered to detect this interaction or the differences in change of mean FSFI score between the two groups. The impact of postsurgical or post-brachytherapy complications were not assessed in this study. Investigation into other variables that may contribute to sexual function in patients with endometrial cancer is necessary. However, these data do provide provocative hypotheses and effect sizes information to guide future power calculations and assessment in larger samples. Larger samples are also needed to provide enough data to assess BMI as a continuous variable and its associations with sexual outcomes.
Review of the Literature
Few studies have looked at sexual health in obese female patients, and even fewer studies have researched sexual health in patients with endometrial cancer treated with VBT. A cross-sectional study of a group of 104 stage I patients treated with simple hysterectomy and VBT assessed sexual dysfunction at less than six months to more than five years post-treatment using the FSFI (Damast et al., 2012). The study found that 81% of patients had sexual dysfunction (FSFI score of 26.55 or less); in addition, FSFI scores across all domains were low. Damast et al. (2012) also found that patients assessed within six months after treatment had lower FSFI scores compared to those assessed at more than six months. The authors concluded that prominent effects of endometrial cancer treatment seen in the initial months after treatment may negatively influence sexual health (Damast et al., 2012). Similarly, sexual function was assessed with the FSFI in a prospective study of 72 patients one to five years postsurgical treatment (13 patients were treated with radiation therapy); in this study, the mean FSFI score was 16.6, and 89% (n = 64) of patients met the criteria for sexual dysfunction based on an FSFI of 26.55 or less at the time of assessment (Onujiogu et al., 2011). A 2014 study by Damast et al. analyzed 255 stage I endometrial cancer survivors at more than one year post-hysterectomy to assess the relationship between post-treatment sexual functioning (using the FSFI) and adjuvant VBT compared to hysterectomy alone. In this sample, 80% of all participants met criteria for sexual dysfunction (as defined by the FSFI) at one to five years post-treatment, regardless of treatment method (Damast et al., 2014). When age and surgery type were controlled for, findings indicated that adjuvant VBT was not associated with sexual function, but laparotomy was (Damast et al., 2014).
Many studies assessing BMI and sexual function have been conducted outside of the United States, so cultural differences related to the attitude toward and reporting of sexual function may exist. A study investigating sexual dysfunction in 45 obese and overweight Turkish women without cancer found no significant difference in total FSFI score between these obese and overweight participants and the 30 menopausal status– and age-matched controls (Yaylali, Tekekoglu, & Akin, 2010). Both groups had sexual dysfunction at the time of assessment, with a mean FSFI score of 22.1 (SD = 4.3) for the obese and overweight group and 23.1 (SD = 3.7) for the control group (Yaylali et al., 2010). Eighty-six percent of the obese and overweight participants and 83% of the control group participants had sexual dysfunction. Comparison of total FSFI scores between the two groups showed no significant difference (p = 0.74) (Yaylali et al., 2010). A case-control study conducted in Italy by Esposito et al. (2007) assessed the association between BMI and female sexual dysfunction in 52 healthy women with female sexual dysfunction (classified as such by an FSFI score of 23 or less) and 66 healthy control women without female sexual dysfunction (an FSFI score of greater than 23) who were matched for age and menopausal status. Among the women with female sexual dysfunction, FSFI scores were significantly lower in the 26 obese and overweight women (BMI of 25 or greater; mean FSFI score of 16.8 [SD = 3.1]) than in the 26 women with a BMI of less than 25 (mean FSFI score of 21.9 [SD = 1]) (p = 0.0001) (Esposito et al., 2007). In the Esposito et al. (2007) study, FSFI score strongly correlated with BMI in otherwise healthy women with female sexual dysfunction (r = –0.72, p = 0.0001).
Obesity and Sexual Dysfunction
Although the current study did not find a significant association between pretreatment BMI and post-treatment sexual function, the data in this small exploratory study did approach significance. Concordant with the current authors’ findings, Yaylali et al. (2010) found that FSFI score did not correlate with BMI and that obesity was not a major contributor to female sexual dysfunction; in addition, Esposito et al. (2007) observed a strong correlation between BMI and FSFI score in women with female sexual dysfunction who were otherwise healthy. Additional research with larger sample sizes is warranted to investigate the association between BMI and female sexual function. It is important to note that, in these studies, the obese and overweight groups had worse sexual function at the time of assessment, even in the presence of female sexual dysfunction. Comparatively, in the current study, the obese and overweight group had low FSFI scores, indicative of sexual dysfunction, at study entry and overall worse sexual function trends with time.
In addition, various reviews of the literature have indicated a strong yet mixed link between sexual function and obesity (Kolotkin et al., 2012; Shah, 2009). Kolotkin et al. (2012) reviewed 47 studies that examined obesity and sexual functioning across genders using diverse methodologies. Their review noted that although various methods, settings, and instruments are used in sexual function research, the link between obesity and sexual function is evident in men and women (Kolotkin et al., 2012). Weight loss studies in men and women (regardless of study design, weight loss methods, and follow-up period) demonstrated improved sexual function with weight reduction; Kolotkin et al. (2012) also determined that many studies of patients with obesity-related comorbidities reveal a connection between obesity and sexual function, regardless of gender.
Three population-based studies analyzed by Kolotkin et al. (2012) included women in the cohort, and two of these studies found that BMI was not significantly linked to sexual function. Studies that involved only female participants had mixed findings compared to the few studies that included male and female participants, which often indicated more sexual dysfunction in women than in men (Kolotkin et al., 2012). Sexual function research has predominately focused on men, particularly with the availability of sildenafil (Viagra®), leaving female sexuality research a largely uncharted territory (Shah, 2009).
Sexual Function and Endometrial Cancer
Very few studies have examined the relationship between BMI and sexual function in endometrial cancer. However, with obesity on the rise and its link to endometrial cancer risk, providers will likely see an increase in endometrial cancer cases (McCullough et al., 2008; Renehan, Tyson, Egger, Heller, & Zwahlen, 2008; Thomas, Wingo, Dolan, Lee, & Richardson, 2009). As of 2016, about 65% of women in the United States were overweight or obese, according to National Health and Nutrition Examination Survey data from 2009–2012 (Mozaffarian et al., 2016). Among U.S. adults, the non-Hispanic Black and Hispanic populations had the highest rates of obese and overweight women (82% and 76%, respectively) compared to non-Hispanic white women (61%) (Mozaffarian et al., 2016). Obese and overweight women in general, but particularly minority women, are at increased risk for the detrimental effects of obesity and its influence on disease states, including endometrial cancer. In addition, with the early detection of endometrial cancer and effective treatment options for endometrial cancer (including VBT), providers will likely see an increase in the number of endometrial cancer survivors. Understanding the various factors that influence the quality of life of endometrial cancer survivors is imperative to improve health outcomes post-treatment.
Other Factors Influencing Sexuality
Female sexuality is multidimensional and influenced by various factors. Distinguishing between female sexual dysfunction and the influence of comorbidities is difficult. Although this study focused only on BMI and sexual function, other factors that may influence sexual function in patients with endometrial cancer must also be considered. Gynecologic cancer and its treatment can affect physical and mental wellness, influencing sexual health, gender identity, and body image through changes in physical appearance, mental health, reproductive ability, menopause, weight, pain, surgical sequelae (e.g., hysterectomy, mastectomy), lack of support, and fatigue (Reis, Beji, & Coskun, 2010; Stilos, Doyle, & Daines, 2008). Additional research is needed in the area of sexual health and gynecologic cancers that is beyond the scope of this study.
Implications for Nursing
Although the current study had a smaller sample size, the authors similarly found that a high percentage of the study group had sexual dysfunction at the time of assessment, regardless of BMI. According to the FSFI, out of the 28 total study participants, 26 had sexual dysfunction at baseline, and 17 had sexual dysfunction at six months. Assessment of mean FSFI score at each time point in the current study showed sexual dysfunction before and within six months after VBT. However, improvements in sexual function were observed between time points; perhaps additional time points more than six months from baseline would allow for better assessment of sexual function in patients with endometrial cancer post-treatment. In addition, this study reiterates the role of obesity in endometrial cancer and its influence on quality of life, particularly post-treatment sexual health. This highlights the opportunity for and impact of nurse-led healthy lifestyle interventions and anticipatory guidance in improving health outcomes in women with endometrial cancer.
The realm of female sexual function remains largely under-researched, despite the high prevalence and complexity of female sexual dysfunction (Kolotkin et al., 2012; Shah, 2009). Sexual function in cancer survivors is complex, and the factors that contribute to dysfunction are multifaceted. Sexuality is an important component of human identity and influences quality of life. A study by Hill et al. (2011) that aimed to identify the sexual healthcare needs of 261 patients with gynecologic and breast cancers found that more than 40% of patients were very interested in receiving care to address sexual issues; however, just 7% of the participants actually received care regarding their sexual health. Impaired sexual function is one aspect of health that affects the quality of life of an estimated 50% of female cancer survivors, challenging healthcare providers to improve regular assessment, treatment, and referral (Krychman & Millheiser, 2013). Nurses can be at the forefront of addressing sexual wellness and providing appropriate interventions regarding sexual health to women with endometrial cancer. For example, nurses can screen for and provide education to patients who may encounter adverse sexual health outcomes post-VBT. The use of the PLISSIT (permission, limited information, specific suggestions, intensive therapy) and BETTER (bring up, explain, tell, timing, educate, record) models have provided a useful framework to help providers address sexual health issues in a patient-centered manner (Annon, 1976; Mick, Hughes, & Cohen, 2003). In addition, nursing interventions aimed at educating and improving the sexual health of endometrial cancer survivors can incorporate healthy weight maintenance as a component. To provide quality nursing care that is holistic and comprehensive, sexual health must be included in the routine care of patients. Nurses spend a majority of their time with patients and are in an optimal position to assess and address sexual health concerns, provide patient education regarding sexual health post-treatment, and refer patients appropriately. Consequently, nurses can spearhead improvements in sexual health among cancer survivors.
Conclusion
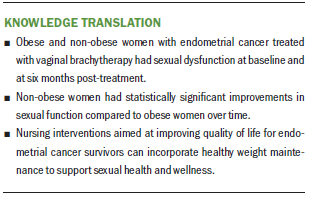
Findings from the current study suggest that obese and non-obese women with endometrial cancer treated with VBT have sexual dysfunction at baseline and at six months post-treatment. However, both groups had an increase in sexual functioning based on mean FSFI score over time. The non-obese women had statistically significant improvements in sexual function compared to obese women from baseline to six months post-treatment; however, neither group reached a level considered to be healthy sexual function. Future work with larger samples is warranted to better understand the impact of BMI on post–cancer treatment sexual function and potential sexual rehabilitation interventions.
About the Author(s)
Rubi M. Garcia, RN, BSN, FNP-C, is a family nurse practitioner in the Nell Hodgson Woodruff School of Nursing at Emory University in Atlanta, GA; Alexandra Hanlon, PhD, is a professor in the School of Nursing at the University of Pennsylvania in Philadelphia; William Small Jr., MD, FACRO, FACR, FASTRO, is a professor and chair of the Department of Radiation Oncology at Loyola University in Chicago, IL; Jonathan B. Strauss, MD, MBA, is an assistant professor in the Feinberg School of Medicine at Northwestern University in Chicago, IL; Lillie Lin, MD, is an associate professor in the Department of Radiation Oncology at the University of Pennsylvania; and Jessica Wells, RN, PhD, is an assistant professor, and Deborah W. Bruner, RN, PhD, FAAN, is a Robert W. Woodruff Professor of Nursing, both in the Nell Hodgson Woodruff School of Nursing at Emory University. This research was funded, in part, by a grant (1R21CA140766-01) from the National Cancer Institute. Small Jr. has previously consulted for Varian Medical Systems and currently receives funding support from Zeiss. Strauss has previously consulted for American Imaging Management and has received payment for services on speakers bureaus from the National Comprehensive Cancer Network and Osler Institute. Lin has previously consulted for and received support from Varian Medical Systems. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Hanlon, Small Jr., and Bruner contributed to the conceptualization and design. Small Jr., Strauss, Lin, and Bruner completed the data collection. Garcia, Hanlon, and Bruner provided statistical support. Garcia, Hanlon, Small Jr., Strauss, Lin, and Bruner provided the analysis. All authors contributed to the manuscript preparation. Garcia can be reached at rubi.garcia@emory.edu, with copy to ONFEditor@ons.org. (Submitted February 2017. Accepted June 27, 2017.)
References
American Cancer Society. (2016a). Endometrial cancer early detection, diagnosis, and staging. Retrieved from https://www.cancer.org/content/dam/CRC/PDF/Public/8611.00.pdf
American Cancer Society. (2016b). Radiation therapy for endometrial cancer. Retrieved from https://www.cancer.org/cancer/endometrial-cancer/treating/radiation.html
Annon, J.S. (1976). The PLISSIT model: A proposed conceptual scheme for the behavioral treatment of sexual problems. Journal of Sex Education and Therapy, 2, 1–15.
Baser, R.E., Li, Y., & Carter, J. (2012). Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer, 118, 4606–4618. https://doi.org/10.1002/cncr.26739
Bergmark, K., Åvall-Lundqvist, E., Dickman, P.W., Henningsohn, L., & Steineck, G. (1999). Vaginal changes and sexuality in women with a history of cervical cancer. New England Journal of Medicine, 340, 1383–1389. https://doi.org/10.1056/NEJM199905063401802
Bruner, D.W., Jones, M., Buchanan, D., & Russo, J. (2006). Reducing cancer disparities for minorities: A multidisciplinary research agenda to improve patient access to health systems, clinical trials, and effective cancer therapy. Journal of Clinical Oncology, 24, 2209–2215. https://doi.org/10.1200/JCO.2005.04.8116
Damast, S., Alektiar, K., Eaton, A., Gerber, N.K., Goldfarb, S., Patil, S., . . . Basch, E. (2014). Comparative patient-centered outcomes (health state and adverse sexual symptoms) between adjuvant brachytherapy versus no adjuvant brachytherapy in early stage endometrial cancer. Annals of Surgical Oncology, 21, 2740–2754. https://doi.org/10.1245/s10434-014-3562-4
Damast, S., Alektiar, K.M., Goldfarb, S., Eaton, A., Patil, S., Mosenkis, J., . . . Basch, E. (2012). Sexual functioning among endometrial cancer patients treated with adjuvant high-dose-rate intra-vaginal radiation therapy. International Journal of Radiation Oncology, Biology, Physics, 84, e187–e193. https://doi.org/10.1016/j.ijrobp.2012.03.030
Esposito, K., Ciotola, M., Giugliano, F., Bisogni, C., Schisano, B., Autorino, R., . . . Giugliano, D. (2007). Association of body weight with sexual function in women. International Journal of Impotence Research, 19, 353–357. https://doi.org/10.1038/sj.ijir.3901548
Fader, A.N., Arriba, L.N., Frasure, H.E., & von Gruenigen, V.E. (2009). Endometrial cancer and obesity: Epidemiology, biomarkers, prevention and survivorship. Gynecologic Oncology, 114, 121–127. https://doi.org/10.1016/j.ygyno.2009.03.039
Ganz, P.A., Rowland, J.H., Desmond, K., Meyerowitz, B.E., & Wyatt, G.E. (1998). Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. Journal of Clinical Oncology, 16, 501–514. https://doi.org/10.1200/JCO.1998.16.2.501
Hill, E.K., Sandbo, S., Abramsohn, E., Makelarski, J., Wroblewski, K., Wenrich, E.R., . . . Lindau, S.T. (2011). Assessing gynecologic and breast cancer survivors’ sexual health care needs. Cancer, 117, 2643–2651. https://doi.org/10.1002/cncr.25832
Ko, E.M., Walter, P., Clark, L., Jackson, A., Franasiak, J., Bolac, C.,. . . Bae-Jump, V.L. (2014). The complex triad of obesity, diabetes and race in Type I and II endometrial cancers: Prevalence and prognostic significance. Gynecologic Oncology, 133, 28–32. https://doi.org/10.1016/j.ygyno.2014.01.032
Kolotkin, R.L., Zunker, C., & Østbye, T. (2012). Sexual functioning and obesity: A review. Obesity, 20, 2325–2333. https://doi.org/10.1038/oby.2012.104
Krychman, M., & Millheiser, L.S. (2013). Sexual health issues in women with cancer. Journal of Sexual Medicine, 10(Suppl. 1), 5–15. https://doi.org/10.1111/jsm.12034
McCullough, M.L., Patel, A.V., Patel, R., Rodriguez, C., Feigelson, H.S., Bandera, E.V., . . . Calle, E.E. (2008). Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiology, Biomarkers and Prevention, 17, 73–79. https://doi.org/10.1158/1055-9965.epi-07-2567
Mick, J., Hughes, M., & Cohen, M.Z. (2003). Sexuality and cancer: How oncology nurses can address it BETTER [Abstract 180]. Oncology Nursing Forum, 30(Suppl. 2), 152–153.
Mozaffarian, D., Benjamin, E.J., Go, A.S., Arnett, D.K., Blaha, M.J., Cushman, M., . . . Turner, M.B. (2016). Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation, 133, e38–e360. https://doi.org/10.1161/CIR.0000000000000350
Must, A., Spadano, J., Coakley, E.H., Field, A.E., Colditz, G., & Dietz, W.H. (1999). The disease burden associated with overweight and obesity. JAMA, 282, 1523–1529. https://doi.org/10.1001/jama.282.16.1523
Onujiogu, N., Johnson, T., Seo, S., Mijal, K., Rash, J., Seaborne, L., . . . Kushner, D.M. (2011). Survivors of endometrial cancer: Who is at risk for sexual dysfunction? Gynecologic Oncology, 123, 356–359. https://doi.org/10.1016/j.ygyno.2011.07.035
Reis, N., Beji, N.K., & Coskun, A. (2010). Quality of life and sexual functioning in gynecological cancer patients: Results from quantitative and qualitative data. European Journal of Oncology Nursing, 14, 137–146. https://doi.org/10.1016/j.ejon.2009.09.004
Renehan, A.G., Tyson, M., Egger, M., Heller, R.F., & Zwahlen, M. (2008). Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet, 371, 569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Rosen, R., Brown, C., Heiman, J., Leiblum, S., Meston, C., Shabsigh, R., . . . D’Agostino, R., Jr. (2000). The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. Journal of Sex and Marital Therapy, 26, 191–208. https://doi.org/10.1080/009262300278597
Rydzewski, N.R., Strohl, A.E., Donnelly, E.D., Kanis, M.J., Lurain, J.R., Nieves Neira, W., & Strauss, J.B. (2016). Receipt of vaginal brachytherapy is associated with improved survival in women with stage I endometrioid adenocarcinoma of the uterus: A National Cancer Data Base study. Cancer, 122, 3724–3731. https://doi.org/10.1002/cncr.30228
Shah, M.B. (2009). Obesity and sexuality in women. Obstetrics and Gynecology Clinics of North America, 36, 347–360. https://doi.org/10.1016/j.ogc.2009.04.004
Small, W., Jr., Beriwal, S., Demanes, D.J., Dusenbery, K.E., Eifel, P., Erickson, B., . . . Gaffney, D. (2012). American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy, 11, 58–67. https://doi.org/10.1016/j.brachy.2011.08.005
Sorosky, J.I. (2012). Endometrial cancer. Obstetrics and Gynecology, 120, 383–397. https://doi.org/10.1097/AOG.0b013e3182605bf1
Stilos, K., Doyle, C., & Daines, P. (2008). Addressing the sexual health needs of patients with gynecologic cancers. Clinical Journal of Oncology Nursing, 12, 457–463. https://doi.org/10.1188/08.CJON.457-463
Thomas, C.C., Wingo, P.A., Dolan, M.S., Lee, N.C., & Richardson, L.C. (2009). Endometrial cancer risk among younger, overweight women. Obstetrics and Gynecology, 114, 22–27. https://doi.org/10.1097/AOG.0b013e3181ab6784
World Health Organization. (n.d.). Sexual and reproductive health: Defining sexual health. Retrieved from http://www.who.int/reproductivehealth/topics/sexual_health/sh_definitio…
Yaylali, G.F., Tekekoglu, S., & Akin, F. (2010). Sexual dysfunction in obese and overweight women. International Journal of Impotence Research, 22, 220–226. https://doi.org/10.1038/ijir.2010.7




