Feasibility and Acceptability of a Dignity Therapy/Life Plan Intervention for Patients With Advanced Cancer
Purpose/Objectives: To determine the feasibility and acceptability of a dignity therapy/life plan intervention in the outpatient oncology setting.
Research Approach: Pilot descriptive study.
Setting: Outpatient clinic in a tertiary oncology center.
Participants: 18 patients within 12 months after diagnosis undergoing treatment for advanced pancreatic cancer or non-small cell lung cancer.
Methodologic Approach: Patients received dignity therapy, consisting of a focused life review/values clarification interview session and two subsequent sessions to produce a generativity document, which they can use later as they wish. Participants also wrote a life plan, in which they listed future hopes and dreams. Intervention feasibility and acceptability for patients and oncology clinician satisfaction were assessed.
Findings: Among the 18 patients completing the intervention, almost all felt it was worthwhile, would do it again, had their expectations met or exceeded, would recommend it to others, and said the timing was just right.
Interpretation: This psychosocial intervention was found to be feasible and acceptable to patients with cancer undergoing active treatment.
Implications for Nursing: Nurses may be in an ideal position to offer a dignity therapy/life plan intervention to patients with advanced cancer during treatment.
Jump to a section
Two of the most physically and psychologically overwhelming cancers are pancreatic and advanced lung cancer. Advanced pancreatic cancer has one of the poorest overall survival rates, with median survival of 10 months or less (American Cancer Society [ACS], 2017; Lazenby & Saif, 2010). Advanced lung cancer is equally devastating and is the primary cause of death from cancer in both men and women, accounting for 27% and 25% of all cancer deaths, respectively (ACS, 2017). For those with clinical stage IV non-small cell lung cancer (NSCLC), median survival is six months (ACS, 2017).
Although high mortality rates and troublesome physical symptoms are demoralizing to those with these cancers, the emotional toll of these illnesses is equally difficult. The term distress encompasses the psychological, social, and spiritual aspects of the emotional experience of cancer and the effect of this experience on coping with the illness and associated symptoms (Holland & Alici, 2010; National Comprehensive Cancer Network, 2016). Unrelieved distress and suffering may lead to loss of meaning and purpose and a hastened desire for death (McClain-Jacobson et al., 2004). Patients with pancreatic and advanced lung cancer experience some of the most severe psychological distress of all patients with cancer (Holland et al., 1986). The prevalence of distress is almost 37% among those with pancreatic cancer and more than 43% among those with lung cancer. These patients also report the highest mean depression and anxiety scores among those with the most common types of cancer (Zabora, BrintzenhofeSzoc, Curbow, Hooker, & Piantadosi, 2001).
Distress also encompasses the concept of loss of dignity, which is defined as being worthy of respect, esteem, and honor (Chochinov, 2012). Loss of dignity has been reported by 46% of those with advanced cancer, and loss of dignity is related to worse quality of life (QOL), hopelessness, depression, and dependency on others for personal care (Hack et al., 2004). Also, dignity has been found to greatly mediate the effect of physical symptoms on demoralization (Vehling & Mehnert, 2014).
Maintaining dignity has been identified as one of the core needs of those with life-threatening illnesses (Meier et al., 2016). Although clinicians continue to seek cure and/or prolong survival for those with pancreatic or advanced lung cancer, equal efforts must be given to relieve suffering and preserve dignity and QOL. Because these patients have potentially shortened life spans, it is imperative that interventions to mitigate distress and promote dignity begin as soon as possible after the cancer diagnosis. Providing supportive palliative care earlier in the cancer trajectory is associated not only with improved QOL but also potentially longer overall survival (National Consensus Project for Quality Palliative Care, 2013; Temel et al., 2010).
Simultaneous incorporation of palliative care interventions that address all domains of QOL into disease-modifying cancer treatment from diagnosis onward is now the standard of care (Ferrell, Temel, Temin, & Smith, 2017; Smith et al., 2012). Therefore, populations with pancreatic and advanced lung cancer may particularly benefit from palliative psychosocial interventions as soon as possible after diagnosis, or at least at disease progression, to alleviate distress, promote dignity, and improve QOL. However, trying to provide psychosocial interventions to patients still undergoing cancer treatment in outpatient settings may be challenging because of scheduling demands associated with active cancer treatment and adverse effects of treatment and disease.
Dignity therapy (DT) is a psychosocial palliative care intervention that can greatly affect QOL but has been used primarily at the end of life. DT was designed as a novel, brief psychotherapeutic intervention, based on an empirically derived model, in which a participant receives a generativity document, the final product of several interview sessions, which he or she can give to family members. This intervention has been found to enhance people’s sense of purpose, meaning, dignity, and overall QOL (Chochinov, 2002, 2007, 2008, 2012; Chochinov et al., 2011). DT research has demonstrated improved overall QOL, dignity, meaning/purpose, sense of generativity and ego-integrity, spiritual well-being, and hope, as well as decreased symptoms of depression and anxiety, in patients with advanced lung, breast, and gastrointestinal cancer at the end of life (Chochinov et al., 2005, 2011; Julião, Oliveira, Nunes, Vaz Carneiro, & Barbosa, 2014; Vuksanovic, Green, Dyck, & Morrissey, 2017). DT participants have reported high rates of satisfaction with the intervention, a greater will to live, decreased sense of suffering, and perceived benefits to the family (Chochinov et al., 2005; Houmann, Chochinov, Kristjanson, Petersen, & Groenvold, 2014; McClement et al., 2007; Montross, Winters, & Irwin, 2011). In a large randomized, controlled trial, patients in palliative care receiving DT were significantly more likely to report the intervention to be helpful than those who received client-centered care or standard palliative care. They also stated that it improved their overall QOL and sense of dignity, promoted spiritual well-being, and lessened sadness (Chochinov et al., 2011). Patients with advanced colorectal cancer reported that DT enhanced their sense of dignity, meaning, purpose, and will to live, and felt that it would be helpful to their families (Vergo, Nimeiri, Mulcahy, Benson, & Emanuel, 2014).
In addition, facing a cancer diagnosis prompts many individuals to clarify their values, determine what is most important to them in life (Hack et al., 2010), and modify life goals. Proactively doing so can positively affect psychosocial well-being. Making a plan or a list of life goals—encompassing physical, emotional, social, and spiritual domains—can influence QOL and the psychosocial impact of illness (Hullmann, Robb, & Rand, 2016; Pinquart, Silbereisen, & Fröhlich, 2009). The reflection that is provided during DT could serve as a form of life review and values clarification, and the development of a generativity document acts as a formal record of both tasks. DT then can become an impetus to develop a life plan (LP)—a list of tasks and/or behaviors patients might want to accomplish—to enhance meaning and purpose in life. This intervention combination has not yet been tested.
Given that DT has been provided in palliative care and end-of-life settings, methods to deliver DT must be adapted to accommodate logistical issues for patients undergoing outpatient cancer treatment. Those providing the intervention must remain sensitive to symptom management issues, busy outpatient practices, and patient perceptions of diagnosis/prognosis. The purpose of this study was to determine the feasibility and acceptability of a DT/LP intervention in the outpatient oncology treatment setting by providing the intervention before the end of life, when it is traditionally offered.
Methods
Design and Setting
This pilot study determined the feasibility and acceptability of the DT/LP intervention and explored study methods in patients with advanced pancreatic or lung cancer receiving treatment. The current authors conducted this study in the outpatient chemotherapy suite at Mayo Clinic in Rochester, Minnesota, while patients were receiving treatment. If radiation therapy was the primary treatment at the time of any DT interviews or data collection time points, DT/LP was conducted in the radiation oncology setting.
Sample and Participant Selection
The sample consisted of adults aged older than 18 years who were diagnosed within the past 12 months and undergoing treatment for advanced pancreatic adenocarcinoma or stage IIIb or IV NSCLC. They had a provider-determined prognosis of six months or more, spoke English, and were cognitively intact, as documented in the electronic health record. They were not currently receiving hospice or formal palliative care services and did not have a concurrent diagnosis of delirium, dementia, major depressive disorder, acute anxiety disorder, or schizophrenia, nor were they participating in other psychosocial intervention research studies. The initial study phase included only patients with pancreatic cancer; the NSCLC group was added later.
Procedures
Approval for the study was obtained from the Mayo Clinic Institutional Review Board for protection of human participants before commencing the study. The authors screened potential participants through the electronic patient appointment system to determine study eligibility. The principal investigator and study coordinator recruited potential participants during treatment appointments and obtained oral consent and a signed Health Insurance Portability and Accountability Act authorization. The first DT session occurred during the patients’ next scheduled treatment appointment (typically two to three weeks later).
The DT/LP intervention was provided by the principal investigator—an advanced practice nurse (APN) who had undergone training in the provision of DT—by following a standard manual of procedures (Chochinov, 2012). The current authors gave participants a slightly modified Dignity Therapy Question Protocol (Chochinov et al., 2005) (see Figure 1) at the baseline recruitment session and asked them to reflect on what they would like to discuss during their initial DT interview session. For the first DT session, the APN met with a patient in the outpatient treatment suite, interviewing him or her using the DT interview guide. Active listening is an integral component of DT, and probing questions were added as needed to clarify and enrich interview content. The interview was planned for 30–60 minutes, on the basis of the patient’s comfort level, and was digitally audio recorded for later transcription. The authors planned to divide the interview into two shorter sessions in the same day, if necessary, depending on patients’ symptoms and energy level. Family member(s) could attend, if the participant desired. At the end of the first session, the authors scheduled a second DT session to occur in person during the next treatment visit or by telephone, if needed. For most treatment regimens, the authors anticipated this occurring two to four weeks later. Interviews were transcribed verbatim, using a secure, web-based transcription service. The interviewer and/or designated editorial assistant then edited the original interview transcript to provide flow and clarity for the first draft of the generativity document. 
The purpose of the second and third DT sessions was to further edit the document with the participant. The APN met with the participant during his or her treatment appointments or by telephone and read the document to the participant while he or she followed along with a copy. The authors also audio recorded the second session to assist and validate further editing. If the session was to take place by telephone, the participant received a copy of the draft by email shortly before the scheduled session. The participant could validate what was discussed at the initial DT session, provide clarification on previous content, and allow additional information to be shared or previous content deleted. Then the authors edited the document as directed by the participant after the second session to provide the final draft for the third DT session.
During the third DT session, the authors provided the final draft of the generativity document to the participant so he or she could make any final changes. If the participant had no modifications, he or she received the final document to keep and share with family members. If changes were needed in the document, the current authors would make final edits and mail it to the participant. The APN then asked the patient to identify three to six items for the LP, on the basis of discussions and values identified during DT, with items loosely structured around the physical, emotional, social, and spiritual domains of QOL. The authors then gave the completed LP to the participant, keeping a copy for their records. Alternatively, if the last two visits were completed by telephone, the authors shared deidentified generativity document drafts by email and mailed final documents to participants.
Data Collection
Demographic data, including age, sex, race, ethnicity, marital status, religious preference, educational level, occupation, primary healthcare insurance, surgical procedure completed (if applicable), cancer staging, treatment regimen, and baseline Eastern Cooperative Oncology Group Performance Scale score, were collected at baseline (Oken et al., 1982). In addition, participants completed outcome measures for QOL, dignity, distress, spirituality, and purpose in life at baseline, at the end of the DT/LP intervention, and three months later, in addition to feasibility/acceptability measures. The purpose of this article is to report only the feasibility and acceptability of the DT/LP intervention.
The current authors measured feasibility by describing the final accrual numbers: the number of patients assessed for eligibility, how many were approached about the study, how many enrolled (and reasons for declining), and how many completed the intervention. They noted patients who were unable to participate because of cognitive decline, who were unable to complete the full protocol and reasons given, who died before completion of the generativity document, and who were lost to follow-up, as well as deviations from the DT protocol and time from DT session 1 to session 3. Patients who left the study after the recruitment session or DT session 1 were considered as having withdrawn, and those who participated in at least two DT sessions were considered as having completed the intervention. The authors also maintained field notes of issues pertaining to feasibility, including those associated with cancer treatment and care processes, and logged the time required to complete surveys, conduct interviews, and edit documents.
Patient acceptability at the final DT visit was measured using the Was It Worth It Questionnaire (Chauhan et al., 2012), a seven-item tool with questions about whether study participation was worthwhile, whether they would participate again, and whether their expectations were met. An investigator-developedDT/LP feedback form was also used to evaluate components such as helpfulness of DT participation in writing the LP list and timing of the intervention in the course of their illness. The authors evaluated oncology clinician satisfaction through a brief emailed satisfaction survey and documented the number of visits by therapists and time taken to deliver the DT/LP intervention.
Findings
Demographics
Eighteen patients completed the DT/LP study, nine in each cancer group (see Table 1). The mean age among both groups was 63.6 years, with a range of 46–78 years in the pancreatic cancer group and 38–77 years in the lung cancer group. The pancreatic sample was predominantly women (eight of nine) and the lung cancer group predominantly men (seven of nine). Participants were mainly White and non-Hispanic. Time since diagnosis was greater for patients with NSCLC, but time in the current study was similar for both groups (5.5 weeks). Treatment regimens were standard practice for these populations.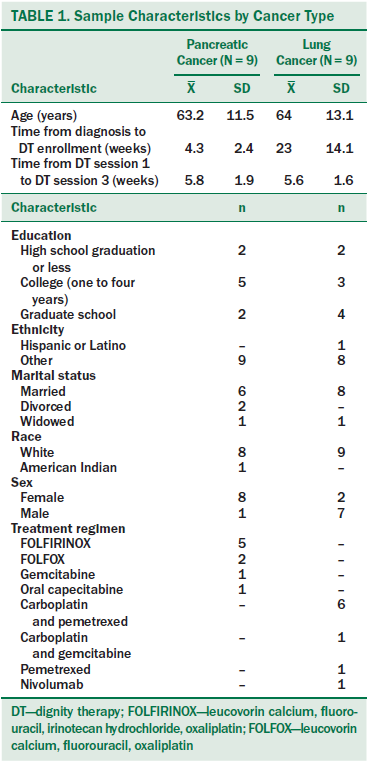
Feasibility
Of 476 electronic health records screened, 431 potential participants were excluded on the basis of preset eligibility criteria (see Figure 2). The most common reason for exclusion was that the patient did not plan to continue treatment at the institution—he or she was there for a second opinion only. Other exclusions were for diagnoses more than 12 months ago and different forms of pancreatic cancer or stage I–IIIA NSCLC. Overall, 45 patients were approached, and 20 declined study participation, most from the NSCLC group.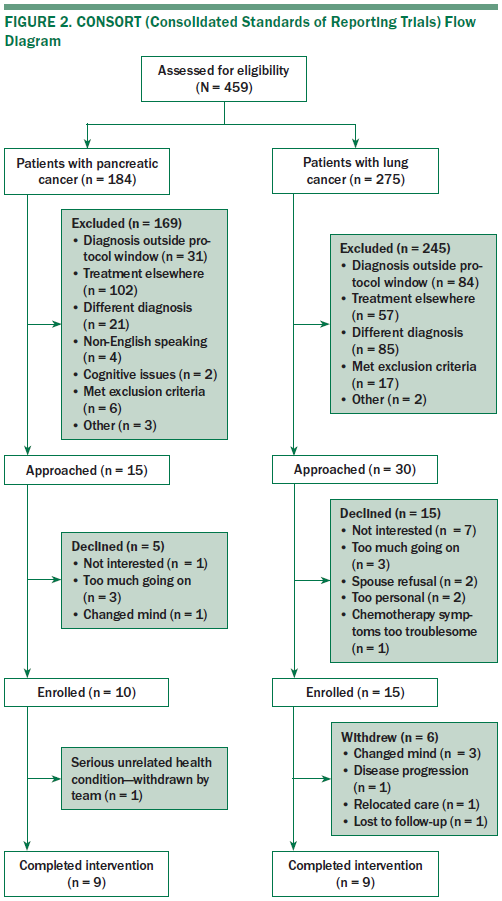
Of those who enrolled, seven withdrew overall, five before they started DT, primarily because they changed their mind. Two withdrew later, one because of a severe automobile accident resulting in a lengthy hospitalization and inability to complete DT/LP and the other was lost to follow-up.
The protocol for provision of DT sessions had some deviations, in which some DT/LP sessions were provided about a week early or a week late. Because treatment schedules varied depending on chemotherapy regimen or delays related to treatment toxicities, the current authors adapted study procedures to meet the overall goal of accommodating treatment schedules. They also were cognizant that treatment needs came first, turning off audio recorders and pausing interviews when medical staff needed to interact with participants (e.g., to change infusion bags, to monitor IV lines).
The initial recruitment visit with the study coordinator included completion of baseline measures and averaged a little longer than one hour. Each participant had three DT visits, and the LP document was completed during the final visit. Each session lasted about an hour and most often occurred in the chemotherapy treatment suite during the participant’s treatment. However, some DT visits and data collection were conducted elsewhere in the clinic. The current authors made use of quiet general access areas (e.g., secluded places in patient lobbies) and meeting rooms near the outpatient chemotherapy suite to promote as much privacy as possible for the patients during interviews.
Initial verification and editing of each transcript to produce a first draft of the generativity document lasted an average of four to five hours. As the authors became more proficient in editing, the study coordinator made initial edits to the verbatim interview transcript before the DT provider edited the first draft for flow, clarity, and cohesiveness, which shortened editing time by two hours. The second and (if needed) third edits to each document did not take as much time, averaging 30 minutes.
Acceptability
Acceptability of the DT/LP intervention was good: All but two participants felt that the intervention was worthwhile, would do it again, had their expectations met or exceeded, and would recommend it to others, as measured by the Was It Worth It Questionnaire (see Table 2). All participants stated that the intervention improved or helped them maintain their QOL. Acceptability reports with the intervention feedback measure provided similar results. Participants reported that the DT intervention helped them develop their LP. Almost all described the timing as just right versus too soon or too late. One participant stated that the intervention was too soon, and one declined to answer the question.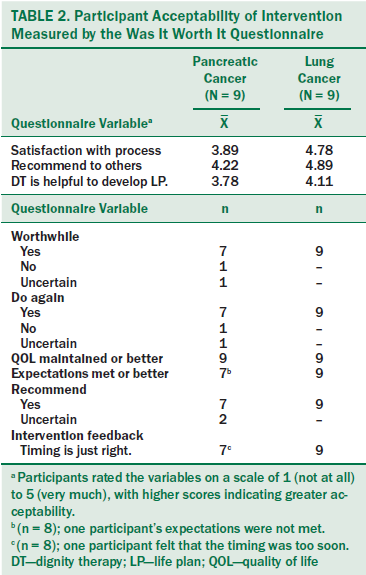
The authors attempted to document oncology provider satisfaction through a brief emailed satisfaction survey but received only four responses. The respondents acknowledged that study participation did not cause additional distress for participants, which was a fear expressed by providers before study initiation. All but one provider noted no disruption in the usual flow of treatment appointments; the one small disruption report occurred early in the study. No further disruptions were noted as the procedures were refined for recruitment and study conduct.
Participants were ready to make their LP lists and completed them in minutes. One participant died a few weeks after his final DT/LP session while fulfilling LP wishes.
Discussion
The DT/LP intervention was feasible and acceptable for patients with advanced pancreatic cancer and NSCLC undergoing active treatment. The premise that conducting the DT intervention first and following it with the LP activity would be feasible and acceptable was also supported. The current authors applied a pragmatic approach to study conduct and modified procedures to fit with patient treatment schedules as much as possible, which supports the emerging literature on DT feasibility in multiple settings, such as in a community hospital–based hospice setting (Montross et al., 2011) and with older adults in residential facilities (Hall, Goddard, Opio, Speck, & Higginson, 2012).
Rather than relying on clinicians to refer patients to this study, the current authors used the electronic appointment scheduling system and screened electronic health records for eligibility. The main reason for study ineligibility was patients did not return to the clinic for subsequent visits. Because the institution is a tertiary referral center, many people come for second opinions only, and clinicians referred patients to settings closer to home if the same treatment was available. The different male:female ratios in the pancreatic and NSCLC groups cannot be explained, because cancer incidence overall is similar in both groups (ACS, 2017). This could have been purely random. Time since diagnosis was longer for participants with NSCLC (mean = 23 weeks versus 4.3 weeks), but the study procedures were modified to allow study entry within the first 12 months of diagnosis and not immediately after diagnosis, as with the pancreatic cancer group, so this was not surprising.
For study feasibility, the authors needed to be flexible in scheduling the DT sessions, based on patients’ return appointments and other treatment follow-up needs, modifying study procedures as necessary, similar to work by Vergo et al. (2014). Particularly for those with NSCLC, who participated later in their disease course, treatment regimens could be changed, and some experienced delays because of treatment toxicities. Scheduling of subsequent DT sessions also required flexibility. They were conducted by telephone or documents emailed for participant review before calls for editing. Although meeting in person would been ideal, not everyone returned for follow-up visits within the study time frame. Modifying conduct of DT sessions did not seem to affect the content of the generativity document, because the authors had established rapport with participants during the initial face-to-face meeting. These study procedure modifications added richness, practicality, and generalizability to the feasibility findings.
Acceptability by patients was high; only two patients with pancreatic cancer were uncertain if they would recommend DT to others. Although patients felt that QOL was maintained or improved, those with pancreatic cancer had a more recent diagnosis than those with NSCLC and may have been still adjusting to the diagnosis and learning to cope with treatment adverse effects (Sohal et al., 2016).
The study completion rate was 90% in both groups once they actually started the intervention, supporting the benefits of offering DT earlier in the disease course. In studies in which DT was offered closer to the end of life, rates of study completion were much lower because of worsening disease or symptoms (Chochinov et al., 2011; Julião et al., 2014; Vergo et al., 2014). DT has been found to be useful in palliative care and end-of-life settings, and palliative care interventions are now recommended earlier in the cancer disease course (Smith et al., 2012).
Limitations
Limitations of the current study were a small sample size and study conduct in a single institution, yet this may be appropriate for a feasibility study. Next research steps should include fully evaluating outcomes from the use of DT/LP with patients with cancer undergoing active treatment, particularly those with poor prognoses and a compressed time frame for psychosocial interventions, to test intervention fidelity with multiple researchers and to explore study conduct across multiple sites.
Implications for Nursing
Nurses may be in an ideal position to offer DT/LP to patients with advanced cancer who are undergoing treatment. The DT provider in this pilot study was a nurse with prior experience administering chemotherapy and conducting symptom management clinical trials. Although nurses are responsible for all aspects of chemotherapy administration, a case could be made for enhancing the nursing role in offering DT/LP to patients with advanced cancer. Nurses possess the leadership, creativity, and critical-thinking skills to advocate for adequate resources to provide psychosocial care concomitantly with cancer treatment. The DT/LP intervention components could be built into the time nurses spend with patients while administering chemotherapy. In providing psychosocial care as a component of whole-person cancer care (Adler & Page, 2008), nurses can enhance overall QOL for patients and their families, which can lead to better treatment outcomes and influence overall survival. In addition, helping patients document their life stories to leave legacies to families can enhance caregiver coping. Finally, a better understanding of all aspects of individuals receiving cancer treatment and hearing their life stories can bring great satisfaction and meaning to oncology nurses.
The incorporation of DT/LP into practice may be particularly feasible for oncology nurses, because patients inherently trust nurses and nursing roles involve incorporating integrative therapies and spiritual interventions into cancer care (Kreitzer & Dose, 2009).
[[{"type":"media","view_mode":"media_original","fid":"34976","attributes":{"alt":"","class":"media-image","height":"212","typeof":"foaf:Image","width":"366"}}]]
Conclusion
The DT/LP intervention was feasible and acceptable to patients with advanced pancreatic cancer or NSCLC while undergoing active treatment. However, administering the intervention early in the disease course required flexibility from the authors because of patients’ schedule changes, because they were still relatively independent and were focused on treatment-related goals and living their lives to the fullest. Additional research is needed regarding implementation of DT combined with LP for patients undergoing disease-modifying treatment for life-limiting illness. Dignity is important to those with cancer but may have a unique meaning to each person. Interventions to promote dignity should be considered part of psychosocial care during treatment to mitigate potential suffering and improve QOL, and should not be reserved for end-of-life situations alone.
About the Author(s)
Dose is a nurse scientist in the Department of Nursing; Hubbard is a medical oncologist and Mansfield is a medical oncologist, both in the Division of Medical Oncology; McCabe is a nurse scientist and Krecke is an associate clinical research coordinator, both in the Department of Nursing; and Sloan is a professor in the Division of Biomedical Statistics and Informatics, all at the Mayo Clinic in Rochester, MN. This research was funded by the Mayo Clinic Saint Mary’s Hospital Sponsorship Board and by a grant (UL1 TR000135) from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health. During the writing of this article, Hubbard was supported by funding from Senhwa Biosciences, Inc., Boston Biomedical, Inc., Genentech, and Merck, and has participated on advisory boards for Genentech and Boehringer Ingelheim. Mansfield has previously consulted for Trovagene Inc., Bristol-Myers Squibb, Genentech, and Celgene and has served on speakers bureaus for Rockpointe. Dose, Hubbard, and Sloan contributed to the conceptualization and design. Dose and Krecke completed the data collection. Dose, McCabe, and Sloan provided statistical support. Dose, Hubbard, Mansfield, McCabe, and Sloan provided the analysis. All authors contributed to the manuscript preparation. Dose can be reached at dose.ann@mayo.edu, with copy to editor at ONFEditor@ons.org. Submitted December 2016. Accepted for publication March 21, 2017.
References
Adler, N.E., & Page, A.E.K. (Eds.) (2008). Cancer care for the whole patient: Meeting psychosocial health needs. Washington, DC: National Academies Press.
American Cancer Society. (2017). Cancer facts and figures, 2017. Retrieved from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and…
Chauhan, C., Atherton, P.J., Satele, D., Dueck, A.C., Soori, G.S., Johnson, D.B., . . . Sloan, J.A. (2012). Patient satisfaction with participation in phase II/III NCCTG clinical trials: Was it worth it? (N0392). Retrieved from http://bit.ly/2uZ1PCC
Chochinov, H.M. (2002). Dignity-conserving care—A new model for palliative care: Helping the patient feel valued. JAMA, 287, 2253–2260.
Chochinov, H.M. (2007). Dignity and the essence of medicine: The A, B, C, and D of dignity conserving care. British Medical Journal, 335, 184–187. doi:10.1136/bmj.39244.650926.47
Chochinov, H.M. (2008). Dignity. Dignity? Dignity! Journal of Palliative Medicine, 11, 674–675. doi:10.1089/jpm.2008.9910
Chochinov, H.M. (2012). Dignity therapy: Final words for final days. Oxford, England: Oxford University Press.
Chochinov, H.M., Hack, T., Hassard, T., Kristjanson, L.J., McClement, S., & Harlos, M. (2005). Dignity therapy: A novel psychotherapeutic intervention for patients near the end of life. Journal of Clinical Oncology, 23, 5520–5525. doi:10.1200/JCO.2005.08.391
Chochinov, H.M., Kristjanson, L.J., Breitbart, W., McClement, S., Hack, T.F., Hassard, T., & Harlos, M. (2011). Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: A randomised controlled trial. Lancet Oncology, 12, 753–762. doi:10.1016/S1470-2045(11)70153-X
Ferrell, B.R., Temel, J.S., Temin, S., & Smith, T.J. (2017). Integration of palliative care into standard oncology care: ASCO clinical practice guideline update summary. Journal of Oncology Practice, 13, 119–121. doi:10.1200/JOP.2016.017897
Hack, T.F., Chochinov, H.M., Hassard, T., Kristjanson, L.J., McClement, S., & Harlos, M. (2004). Defining dignity in terminally ill cancer patients: A factor-analytic approach. Psycho-Oncology, 13, 700–708. doi:10.1002/pon.786
Hack, T.F., McClement, S.E., Chochinov, H.M., Cann, B.J., Hassard, T.H., Kristjanson, L.J., & Harlos, M. (2010). Learning from dying patients during their final days: Life reflections gleaned from dignity therapy. Palliative Medicine, 24, 715–723. doi:10.1177/0269216310373164
Hall, S., Goddard, C., Opio, D., Speck, P., & Higginson, I.J. (2012). Feasibility, acceptability and potential effectiveness of dignity therapy for older people in care homes: A phase II randomized controlled trial of a brief palliative care psychotherapy. Palliative Medicine, 26, 703–712. doi:10.1177/0269216311418145
Holland, J.C., & Alici, Y. (2010). Management of distress in cancer patients. Journal of Supportive Oncology, 8, 4–12.
Holland, J.C., Korzun, A.H., Tross, S., Silberfarb, P., Perry, M., Comis, R., & Oster, M. (1986). Comparative psychological disturbance in patients with pancreatic and gastric cancer. American Journal of Psychiatry, 143, 982–986. doi:10.1176/ajp.143.8.982
Houmann, L.J., Chochinov, H.M., Kristjanson, L.J., Petersen, M.A., & Groenvold, M. (2014). A prospective evaluation of dignity therapy in advanced cancer patients admitted to palliative care. Palliative Medicine, 28, 448–458. doi:10.1177/0269216313514883
Hullmann, S.E., Robb, S.L., & Rand, K.L. (2016). Life goals in patients with cancer: A systematic review of the literature. Psycho-Oncology, 25, 387–399. doi:10.1002/pon.3852
Julião, M., Oliveira, F., Nunes, B., Vaz Carneiro, A., & Barbosa, A. (2014). Efficacy of dignity therapy on depression and anxiety in Portuguese terminally ill patients: A phase II randomized controlled trial. Journal of Palliative Medicine, 17, 688–695. doi:10.1089/jpm.2013.0567
Kreitzer, M.J., & Dose, A.M. (2009). The role of spirituality. In D.I. Abrams & A.T. Weil (Eds.), Integrative oncology (pp. 385–396). New York, NY: Oxford Press.
Lazenby, J.M., & Saif, M.W. (2010). Palliative care from the beginning of treatment for advanced pancreatic cancer: Highlights from the 2010 ASCO Gastrointestinal Cancers Symposium. Orlando, FL,. Journal of the Pancreas, 11, 154–157.
McClain-Jacobson, C., Rosenfeld, B., Kosinski, A., Pessin, H., Cimino, J.E., & Breitbart, W. (2004). Belief in an afterlife, spiritual well-being and end-of-life despair in patients with advanced cancer. General Hospital Psychiatry, 26, 484–486. doi:10.1016/j.genhosppsych.2004.08.002
McClement, S., Chochinov, H.M., Hack, T., Hassard, T., Kristjanson, L.J., & Harlos, M. (2007). Dignity therapy: Family member perspectives. Journal of Palliative Medicine, 10, 1076–1082. doi:10.1089/jpm.2007.0002
Meier, E.A., Gallegos, J.V., Thomas, L.P.M., Depp, C.A., Irwin, S.A., & Jeste, D.V. (2016). Defining a good death (successful dying): Literature review and a call for research and public dialogue. American Journal of Geriatric Psychiatry, 24, 261–271. doi:10.1016/j.jagp.2016.01.135
Montross, L., Winters, K.D., & Irwin, S.A. (2011). Dignity therapy implementation in a community-based hospice setting. Journal of Palliative Medicine, 14, 729–734. doi:10.1089/jpm.2010.0449
National Comprehensive Cancer Network. (2016). NCCN distress thermometer and problem list for patients. Retrieved from https://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distr…
National Consensus Project for Quality Palliative Care. (2013). Clinical practice guidelines for quality palliative care, third edition. Retrieved from https://www.hpna.org/multimedia/NCP_Clinical_Practice_Guidelines_3rd_Ed…
Oken, M.M., Creech, R.H., Tormey, D.C., Horton, J., Davis, T.E., McFadden, E.T., & Carbone, P.P. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology, 5, 649–655.
Pinquart, M., Silbereisen, R.K., & Fröhlich, C. (2009). Life goals and purpose in life in cancer patients. Supportive Care in Cancer, 17, 253–259. doi:10.1007/s00520-008-0450-0
Smith, T.J., Temin, S., Alesi, E.R., Abernethy, A.P., Balboni, T.A., Basch, E.M., . . . Von Roenn, J.H. (2012). American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. Journal of Clinical Oncology, 30, 880–887. doi:10.1200/JCO.2011.38.5161
Sohal, D.P.S., Mangu, P.B., Khorana, A.A., Shah, M.A., Philip, P.A., O’Reilly, E.M., . . . Laheru, D. (2016). Metastatic pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology, 34, 2784–2796. doi:10.1200/JCO.2016.67.1412
Temel, J.S., Greer, J.A., Muzikansky, A., Gallagher, E.R., Admane, S., Jackson, V.A., . . . Lynch, T.J. (2010). Early palliative care for patients with metastatic non–small-cell lung cancer. New England Journal of Medicine, 363, 733–742. doi:10.1056/NEJMoa1000678
Vehling, S., & Mehnert, A. (2014). Symptom burden, loss of dignity, and demoralization in patients with cancer: A mediation model. Psycho-Oncology, 23, 283–290. doi:10.1002/pon.3417
Vergo, M.T, Nimeiri, H., Mulcahy, M., Benson, A., & Emanuel, L. (2014). A feasibility study of dignity therapy in patients with stage IV colorectal cancer actively receiving second-line chemotherapy. Journal of Community Supportive Oncology, 12, 446–453.
Vuksanovic, D., Green, H.J., Dyck, M., & Morrissey, S.A. (2017). Dignity therapy and life review for palliative care patients: A randomized controlled trial. Journal of Pain and Symptom Management, 53, 162–170. doi:10.1016/j.jpainsymman.2016.09.005
Zabora, J., BrintzenhofeSzoc, K., Curbow, B., Hooker, C., & Piantadosi, S. (2001). The prevalence of psychological distress by cancer site. Psycho-Oncology, 10, 19–28.




