Measurement of Hyperglycemia and Impact on Health Outcomes in People With Cancer: Challenges and Opportunities
Problem Identification: Poor health outcomes have been associated with hyperglycemia in patients with and without diabetes. However, the impact of hyperglycemia on the health-related outcomes of patients with cancer has shown conflicting results. The purpose of this review was to explore definitions and measurement issues related to the assessment of hyperglycemia and the subsequent impact on the findings of health-related outcomes in adults with cancer.
Literature Search: Four electronic databases were searched: MEDLINE®, PubMed, CINAHL®, and Web of Science. The search terms were cancer, hyperglycemia, measurement, adults, and health-related outcomes. Only quantitative manuscripts were reviewed. Articles that focused globally on diabetes, hyperglycemia, and/or cancer that did not discuss health-related outcomes were excluded from this review.
Data Evaluation: A total of 30 articles were reviewed. Quantitative articles were synthesized using integrative review strategies.
Synthesis: Three key gaps were identified in the literature: variations in the calculation of hyperglycemia prevalence and in the measurement of hyperglycemia, as well as inconsistent use of standard guidelines.
Conclusions: This review highlights the inconsistencies in measuring or assessing hyperglycemia and the lack of standardized guidelines in treating hyperglycemia. Failure to have a standard approach to the measurement and management of hyperglycemia impedes the ability of healthcare providers to determine the significance of its impact on health outcomes. Further research is needed to establish appropriate measurement guidelines to address hyperglycemia in people with cancer.
Implications for Practice: Evidence-based measurement and treatment guidelines are needed to inform and assist healthcare providers with clinical decision making for people with cancer who experience hyperglycemia.
Jump to a section
Hyperglycemia, an elevation in blood glucose, is a major side effect of cancer and its treatment. In patients with cancer, hyperglycemia frequently occurs independent of the diagnosis of diabetes (Farrokhi, Smiley, & Umpierrez, 2011). Among patients with various types of cancer, the prevalence of hyperglycemia ranges from 39%–99% (Hammer et al., 2009; Karnchanasorn, Malamug, Jin, Karanes, & Chiu, 2012; Storey & Von Ah, 2015).
Among critically and noncritically ill patients, hyperglycemia has been associated with infection and sepsis, stroke, hemorrhage, ileus, and venous thromboembolism (Jiménez-Ibáñez, Castillejos-López, Hernández, Gorocica, & Alvarado-Vásquez, 2012; Mraovic et al., 2010; Zuurbier et al., 2016); longer hospital length of stay (Masrur et al., 2015); and increased morbidity and mortality (Egi et al., 2008; Hermanides et al., 2010). Harmful consequences of hyperglycemia have also been noted among patients with cancer (Storey & Von Ah, 2012, 2015). In preclinical and clinical studies, hyperglycemia has been shown to heighten the risk for the development and progression of cancer, affect diagnostic imaging studies, attenuate the immune system, and increase resistance to chemotherapy (Adham et al., 2014; Biernacka et al., 2013; Germenis & Karanikas, 2007; Harris et al., 2013; Kellenberger et al., 2010; Rabkin, Israel, & Keidar, 2010). However, researchers have reported discordant findings related to the impact of hyperglycemia on health-related outcomes, including infection, toxicity, morbidity, and/or mortality (Olausson, Hammer, & Brady, 2014; Storey & Von Ah, 2012). The inconsistency in findings from these studies may be attributed to type of cancer and/or treatment, patient characteristics, and/or clinical factors. In addition, variations in the definition and measurement of hyperglycemia may also result in ambiguous findings.
The American Diabetes Association ([ADA], 2016) has proposed general guidelines for glycemic thresholds and standards for treatment. However, people with cancer experience unique challenges to glycemic homeostasis because of the disease and its treatment (Hammer et al., 2016). Factors contributing to hyperglycemia in people with cancer include physiologic stress, glycemic status (glucose intolerant, diagnosis of diabetes, diagnosis of prediabetes, or unknown diagnosis of diabetes), the administration of glucocorticoids, and changes in nutrition and activity (Dungan, Braithwaite, & Preiser, 2009; Harris et al., 2013; Hershey et al., 2014). An additional challenge is the receipt of frequent blood transfusions to address disease- and treatment-related anemia, which precludes the use of the hemoglobin A1c (HbA1c) test, the blood test that is recommended for diagnosing diabetes and monitoring blood glucose (Farrokhi et al., 2011; Schrot, Patel, & Foulis, 2007). These challenges present a formidable task for clinicians in trying to determine the best treatment plan to manage hyperglycemia in people with cancer. In addition, the impact of hyperglycemia on subsequent health outcomes may not be fully appreciated because of these variations and, ultimately, could result in the inappropriate treatment of hyperglycemia in clinical practice.
The wide variation in the reported prevalence of hyperglycemia makes ascertaining the severity of the problem in people with cancer difficult. The ramifications of hyperglycemia on the progression, treatment, and response of cancer, as well as the subsequent inconsistencies reported in health outcomes, are important to understand. Identifying glycemic thresholds and measurement issues is imperative to provide clinicians with evidence on which to base treatment decisions, improving care and outcomes for people with cancer.
The purpose of this review was to explore definitions and measurement issues related to the assessment of hyperglycemia and the subsequent impact of these findings on health-related outcomes in adults with cancer. Findings will be used to elucidate gaps related to clinically meaningful measurement issues in patients with cancer. This will, in turn, propel further research that focuses on identifying high-risk patients and inform the development of standardized criteria to evaluate and treat hyperglycemia in adults with cancer.
Methods
Four electronic databases were searched, and articles focusing on hyperglycemia, cancer, and health-related outcomes were targeted. The databases searched were MEDLINE®, PubMed, CINAHL®, and Web of Science. The search terms used were cancer, hyperglycemia, measurement, adults, and health-related outcomes. To best evaluate the definitions and measurement processes related to hyperglycemia and the subsequent impact, health-related outcomes were included in the search terms. These were used as keywords and as medical subject heading (MeSH) terms to obtain as many publications as possible. Reference lists were also searched for pertinent publications. Inclusion criteria were manuscripts that reportedfindings from quantitative research that were published in English from 2004–2015. Figure 1 is a diagram of the search strategy.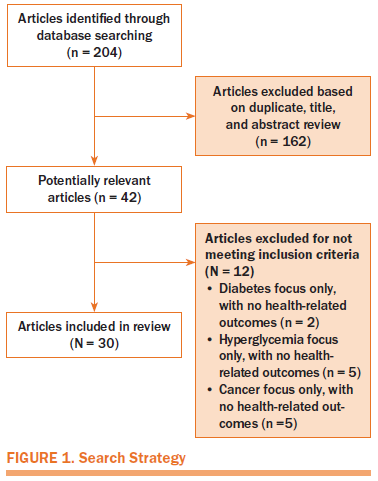
For this review, hyperglycemia was defined as an elevation in blood glucose as defined by each study. Health-related outcomes were also defined by study parameters. Quantitative studies that focused globally on diabetes, hyperglycemia, and/or cancer but did not discuss hyperglycemia and its impact on specific health-related outcomes were excluded. In addition, studies that included hyperglycemia in children with cancer were also excluded because the intent of this review was to examine the blood glucose levels and health-related outcomes of adults with cancer.
The synthesis of quantitative articles was conducted using the integrative methodology strategies proposed by Whittemore and Knafl (2005). These strategies offer a structured format in which at least two researchers conduct an independent review of the articles. If discrepancies occurred, the review was conducted jointly until consensus was reached.
Synthesis
A total of 30 studies met inclusion criteria. The majority of the studies used a retrospective design (n = 26), three were prospective, and one was a case-control study. People receiving stem cell or bone marrow transplantation were the most studied population, appearing in 12 studies. Other populations included those with solid tumors, hematologic cancers, or multiple diagnoses. Table 1 presents the key characteristics and findings of quantitative studies that examined the parameters used to measure hyperglycemia and the health-related outcomes associated with hyperglycemia in people with cancer.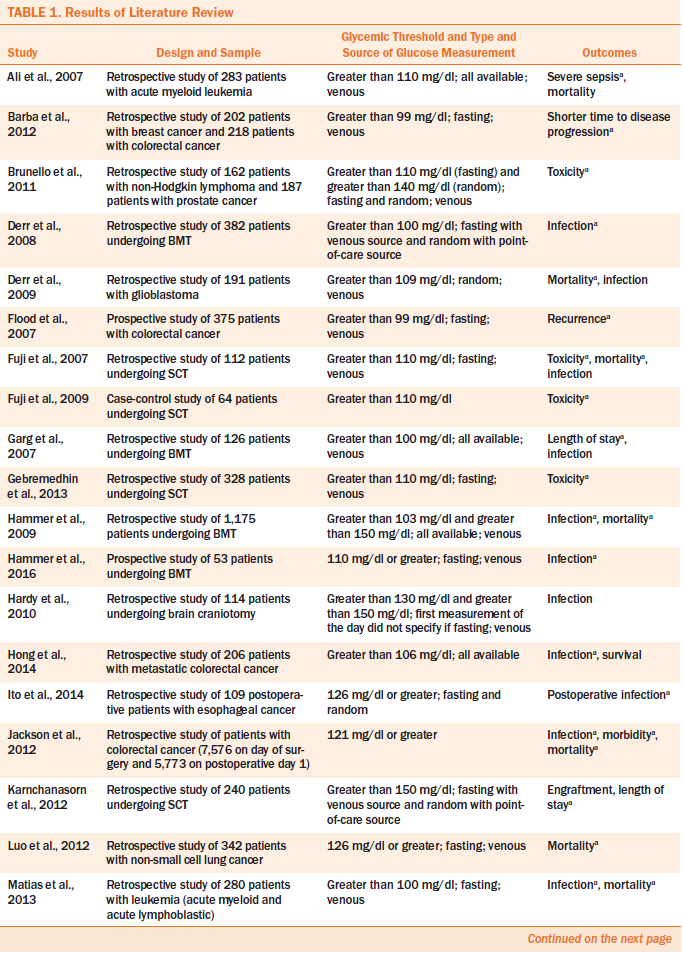
Prevalence
The occurrence of hyperglycemia among people with cancer was reported (as percent of patients above the established threshold) in 7 of the 30 studies (Ali et al., 2007; Hammer et al., 2009; Luo, Chen, & Chang, 2012; Matias, Lima, Teixeira, Souto, & Magalhães, 2013; Rentschler et al., 2010; Storey & Von Ah, 2015; Weiser et al., 2004). Only one study reported a calculation of prevalence and provided the formula (Hardy, Nowacki, Bertin, & Weil, 2010).
Glycemic Measurement Issues
Glycemic threshold: Thresholds used to define hyperglycemia varied among the 30 studies. Thirteen studies used a threshold ranging from 99 mg/dl to 125 mg/dl, seven used a threshold greater than or equal to 126 mg/dl, and two studies used a threshold greater than 200 mg/dl. In addition, six studies used multiple thresholds to define hyperglycemia. Two studies categorized elevated blood glucose values into quartiles (beginning at 106 mg/dl) (Hong et al., 2014) or ranges, such as mild, moderate, and severe, beginning at 121 mg/dl (Jackson, Amdur, White, & Macsata, 2012).
The ADA (2016) criteria for diagnosing prediabetes and diabetes was used in seven of the studies. Two studies used the ADA criteria for diagnosing prediabetes (greater than 100 mg/dl) (Matias et al., 2013; Wright et al., 2013). Four studies used the ADA threshold (126 mg/dl or greater) for diagnosing diabetes (Ito et al., 2014; Luo et al., 2012; Storey & Von Ah, 2015, 2016). One study used the ADA thresholds for the diagnosis of diabetes (fasting blood glucose [FBG] of 126 mg/dl or greater, or random blood glucose of 200 mg/dl or greater) (Rentschler et al., 2010).
Type of glucose measurement: Although 12 studies used FBG only (Barba et al., 2012; Flood et al., 2007; Fuji et al., 2007; Gebremedhin, Behrendt, Nakamura, Parker, & Salehian, 2013; Hammer et al., 2016; Luo et al., 2012; Matias et al., 2013; Sheean, Freels, Helton, & Braunschweig, 2006; Sheean, Kilkus, Liu, Maciejewski, & Braunschweig, 2013; Storey & Von Ah, 2015, 2016; Villarreal-Garza et al., 2012), a variety of testing types were used in the studies. Three studies used random blood glucose only (Derr et al., 2009; Soysal et al., 2012; Tieu et al., 2015), five did not specify (Fuji et al., 2009; Hardy et al., 2010; Jackson et al., 2012; Weiser et al., 2004; Wright et al., 2013), three used a combination of FBG and random venous blood glucose tests (Brunello, Kapoor, & Extermann, 2011; Pidala et al., 2011; Rentschler et al., 2010), and six used all available blood glucose values, including point-of-care (POC) testing in some studies (Ali et al., 2007; Derr, Hsiao, & Saudek, 2008; Garg, Bhutani, Alyea, & Pendergrass, 2007; Hammer et al., 2009; Hong et al., 2014; Karnchanasorn et al., 2012). One study used fasting and random blood glucose drawn every 12 hours but did not specify whether the source was venous or capillary via POC testing (Ito et al., 2014).
Source of glucose measurement: The majority of studies (n = 21) identified the source of blood glucose measurement as blood drawn only from venous sites and analyzed by the hospital laboratory. Six studies did not specify the source of the blood glucose sample, and two studies used venous and capillary blood glucose values taken from POC monitors. One study (Hong et al., 2014) reported using all available glucose measurements without defining what those measurements included. None of the studies in patients with cancer examined whether the source of the blood used for the measurement affected health-related outcomes.
Timing of glucose measurement in the cancer treatment trajectory: Almost half of the studies reviewed examined the timing of hyperglycemia at different intervals throughout the trajectory of cancer and its treatment. Two studies examined the impact of hyperglycemia at the time of diagnosis on disease progression (Barba et al., 2012) and recurrence (Wright et al., 2013), respectively. One study looked at the impact of hyperglycemia using FBG on outcomes at three time points (at baseline and at one year and four years postdiagnosis) in the trajectory of colorectal cancer (Flood et al., 2007). Five studies observed the impact of hyperglycemia in people with hematologic cancers before, during, and/or after the neutropenic period (which is the time in treatment where the white blood cell count is lowest) (Derr et al., 2008; Fuji et al., 2007; Matias et al., 2013; Storey & Von Ah, 2015, 2016). Also assessed was the glycemic status from day 0 (receipt of hematopoietic cells) to day 100 (Hammer et al., 2009), as well as daily morning fasting glycemic status from admission to discharge or 28 days post-transplantation, whichever came first (Hammer et al., 2016). The effects of hyperglycemia on postoperative outcomes were examined, too. For instance, Ito et al. (2014) assessed blood glucose every 12 hours for the first 72 hours postoperation for esophageal cancer, followed by every 24 hours thereafter to the seventh day postoperation. Outcomes of hyperglycemia in patients with colorectal cancer were studied on the operative day and postoperative day 1 by Jackson et al. (2012).
Patterns of hyperglycemia: Only six studies included the number of times an elevation in blood glucose was required to be defined as hyperglycemia (Hammer et al., 2009; Matias et al., 2013; Rentschler et al., 2010; Storey & Von Ah, 2015, 2016; Weiser et al., 2004). However, these requirements varied among the studies. Four studies stipulated that one or more episodes were considered to be hyperglycemia (Hammer et al., 2009; Matias et al., 2013; Storey & Von Ah, 2015, 2016). Two studies classified two or more episodes as hyperglycemia but did not specify the pattern (random or consecutive) of occurrence (Rentschler et al., 2010; Weiser et al., 2004).
Inconsistent Use of Guidelines
The majority of studies (n = 19) did not identify the guidelines on which they based the definition used as the threshold for hyperglycemia. Four authors identified the use of guidelines from empirical research conducted in critical care patients to inform their definition of hyperglycemia (Ali et al., 2007; Hammer et al., 2016; Sheean et al., 2006; Soysal et al., 2012). Five studies used the ADA (2016) guidelines to define hyperglycemia, which is in accordance with the ADA criteria for diagnosis of diabetes (Ito et al., 2014; Luo et al., 2012; Rentschler et al., 2010; Storey & Von Ah, 2015, 2016). One study identified the use of ADA guidelines for categorizing hyperglycemia into groups: impaired glucose for greater than 100 mg/dl and diabetes for greater than 126 mg/dl (Wright et al., 2013). One study defined hyperglycemia as greater than 100 mg/dl based on the ADA’s definition for impaired glucose tolerance (Matias et al., 2013). Gallo, Gentile, Arvat, Bertetto, and Clemente (2016) suggested that the lack of glycemic guidelines for people with cancer, diabetes, and/or hyperglycemia results in a trial-and-error approach that culminates in substandard disease management.
Health-Related Outcomes
The health-related outcomes examined in the studies were reviewed for association with hyperglycemia. The association between hyperglycemia and toxicity was found in each of the six studies that assessed this outcome. Various studies also determined a link between hyperglycemia and survival, disease progression, and recurrence. However, incongruent findings among the studies were found related to the impact of hyperglycemia on other health-related outcomes of patients with cancer. Mortality was found to be associated with hyperglycemia in 9 of 10 studies that examined this outcome. Equivocal findings were noted among studies that examined infection, with some finding an association and others not. Inconsistent findings related to hyperglycemia and length of stay were found, with some showing a relationship and others not. A few of the studies examined health-related outcomes that were not assessed in the other studies, such as morbidity, engraftment, number of neutropenic days, and complete remission, which prevented comparison. The disparity in these findings may be attributable to measurement issues.
Discussion
The purpose of this integrative review was to explore and critically appraise the empirical literature related to the definitions and measurement of hyperglycemia and the subsequent impact of these findings on health-related outcomes in adults with cancer. A total of 30 quantitative studies were reviewed. Three key gaps were identified among the studies: (a) variations in the calculation of hyperglycemia prevalence, (b) variations in the measurement of hyperglycemia, and (c) inconsistent use of standard guidelines.
Most studies in this review reported a wide range of percentages of patients with hyperglycemia. Only one of the studies reported prevalence and disclosed the formula used for calculation. The studies reviewed noted ambiguity regarding the deleterious impact of hyperglycemia on health-related outcomes. These discrepancies may be attributed to the various thresholds used and the lack of a standard formula for calculating and reporting prevalence. Inconsistency in measurement and reporting may result in under- or overtreatment of hyperglycemia. To better comprehend the magnitude of hyperglycemia in people with cancer, a standard threshold and formula for calculating prevalence is needed.
The type of blood glucose measurement may be important to examine as a predictor of poor outcomes. Epidemiologic studies conducted in people without cancer have shown that in patients with and without diabetes, elevated FBG is associated with an increased risk for vascular disease (Sarwar et al., 2010), whereas elevations in postprandial or random blood glucose are associated with morbidity (Cavalot et al., 2006) and mortality (Umpierrez et al., 2002), respectively. Twelve studies in this review used FBG only to determine hyperglycemia; however, inconsistencies existed regarding the type of glucose measurement used in these studies. People with cancer often receive glucocorticoids as part of their treatment regimen, which can contribute to exaggerations in blood glucose. Frequent monitoring for glucocorticoid-induced hyperglycemia is recommended. FBG may not adequately reflect the influence of glucocorticoids because postprandial (two hours postmeal) and random blood glucose (four to six hours after receipt of glucocorticosteroids) best capture their effects on blood glucose (Gallo et al., 2016; Harris et al., 2013). In the studies reviewed, the type of glucose measurement reported may have been influenced by known diabetes status and/or practitioner preference. Additional research is needed to identify which people are at high risk for hyperglycemia and to develop methods to screen for these high-risk patients throughout the cancer trajectory. In addition, ascertaining when blood glucose should be monitored and establishing tolerable thresholds is important.
The majority of studies in this review used the blood glucose results from venous samples analyzed by the laboratory. Laboratory-analyzed venous blood sampling is considered to be the most accurate source of measurement (Farmer, 2010); however, these results are only a measurement of blood glucose at the time the blood was obtained and may not reflect the actual patient experience. POC testing is most often used and is the standard of care in the hospital setting for frequent monitoring of blood glucose. The advantage of POC testing is that it provides a real-time analysis of blood glucose. However, POC devices can vary by more than 30% for glucose levels greater than 150 mg/dl and by 60% for those in the hypoglycemic range (Dungan, Chapman, Braithwaite, & Buse, 2007). Certain metabolic factors (e.g., inadequate tissue perfusion, hypotension, hypoxia, anemia) can also alter POC results in people with cancer (Hermayer et al., 2015; Hirsch, 2010). POC testing can also be contraindicated because of the risk for infection with invasive venipuncture or finger sticks to vulnerable immunosuppressed people with cancer.
Most of the studies in this review used FBG as the measure for blood glucose. Unlike studies in other populations, none of the studies reviewed reported the use of the HbA1c test. This test, a measure of blood glucose over time, is not regularly used in people with cancer because of its dependence on the normal function and lifespan of red blood cells. The use of blood transfusions and/or the diagnosis of anemia alter results of the HbA1c test, leading to inaccurate results and potentially inappropriate treatment (Oyer, Shah, & Bettenhausen, 2006). No recommendations exist for the most appropriate source of blood sampling in hospitalized people with cancer. The use of serum and/or POC blood glucose alone or in combination with other measures of hyperglycemia is important to study to facilitate standardization of practice and management. More research is needed to determine if the use of FBG, postprandial blood glucose, and/or random blood glucose alone or in combination should be used to detect hyperglycemia in people with cancer. In addition, the use of POC testing, in conjunction with other types of glucose measurements to identify hyperglycemia, and its impact on health-related outcomes require further exploration.
Fructosamine and/or glycated albumin may be an alternative measurement of hyperglycemia for select people for whom the HbA1c test may be deemed unreliable (Danese, Montagnana, Nouvenne, & Lippi, 2015; Malmström et al., 2014). More research is needed to determine the effectiveness of alternative glucose measures in detecting hyperglycemia in people with cancer. In addition, noninvasive continuous glucose monitoring devices, which are in development, are a promising option that may be beneficial in assessing hyperglycemia in people with immune suppression.
Most studies in this review did not use or indicate the guidelines on which their selected threshold was based; as a result, various thresholds were used to define hyperglycemia. Of the studies that used guidelines, the sources varied between those using empirical findings from critical care populations and those using ADA guidelines to determine the definition of hyperglycemia. The ADA guidelines are the standard of care for the treatment of hyperglycemia in hospitalized patients. Many hospitals use evidence-based protocols that include the ADA guidelines, which provide recommendations to define and guide the treatment of hyperglycemia. However, in most empirical research studies reviewed, these guidelines were not being used to establish thresholds, with no explanation of this rationale. An additional challenge with use of the ADA guidelines is that different guidelines exist for hyperglycemia dependent on etiology (induced by diabetes, stress, or steroids). Whether these guidelines are adequate and/or appropriate for people with cancer, or whether specific guidelines should be established for this unique population of patients, has yet to be determined.
Among the studies, the timing and frequency by which blood glucoses were obtained could have varied by degree of illness or diabetic status, with those people with higher acuity and known diabetes being monitored more closely. Some of the studies described the timing of glycemic measurements in the cancer trajectory and its impact on health-related outcomes. Time points studied included baseline (prior to treatment), postoperation, and before and during the neutropenic period and/or at intervals. These studies revealed that hyperglycemia during these time periods was associated with poor health outcomes (disease progression, recurrence, survival, graft-versus-host disease, infection, and mortality). Although the studies were conducted among heterogeneous patient populations, they suggest that the onset of hyperglycemia at different time points in the cancer trajectory may influence health outcomes. More research is warranted to determine if there are critical time periods during which the onset of hyperglycemia is more deleterious to inform preventive and screening strategies to identify patients at high risk.
The impact of hyperglycemia on the health outcomes of patients with cancer cannot be fully elucidated until these gaps are addressed. These gaps challenge the appropriateness of current guidelines and suggest that standard guidelines for glycemic thresholds and measurement processes should be identified and implemented to address the unique needs of people with cancer who experience hyperglycemia. The impact of hyperglycemia on health outcomes can then be more thoroughly assessed while reducing the influence of these confounding issues.
Limitations
To the current authors’ knowledge, this is the first review summarizing the empirical research from quantitative studies examining the gaps in measurement thresholds of hyperglycemia in people with cancer. The lack of clear and common thresholds and measurement processes makes comparison across studies difficult. Because of discrepancies in the reporting of prevalence and thresholds used, as well as variations in timing, frequency, and sources used for obtaining blood samples, the ability to assess the negative effects of hyperglycemia on health-related outcomes is limited. This review elucidates the need for well-defined thresholds and standards of practice for glycemic management in people with cancer that may influence health-related outcomes.
Implications for Research
Additional variables may contribute to elevation in blood glucose. Host characteristics, such as unknown or prediabetes status, can contribute to hyperglycemia. In the United States, about 8.1 million people with diabetes do not know they have the disease. An estimated 37% of adults have prediabetes (an elevation in blood glucose that is higher than normal but not high enough to be considered diabetes), with only 11% being aware that they are in this category (Centers for Disease Control and Prevention, 2014). Treatment characteristics, such as the disease process and associated stressors, may influence hyperglycemia. In addition, the administration of certain chemotherapies and biologic agents and glucocorticosteroids in many treatment regimens may potentiate hyperglycemia in people with cancer, with and without a diagnosis of diabetes. Behavioral characteristics, such as nutritional intake, activity, and other lifestyle factors that may be altered because of illness, can play a role in hyperglycemia. Among hospitalized people with cancer, on-call meal services common in many hospitals contribute to challenges in obtainingaccurate morning FBG levels and subsequent glycemic management. Future research should examine the influence of these factors on hyperglycemia and health-related outcomes in people with cancer.
Understanding hyperglycemia is important because it may be indicative of underlying glucose intolerance, particularly when it occurs in nondiabetic people with cancer; if untreated, it can result in chronic glucose dysfunction and/or diabetes (Falciglia, 2007; Gallo et al., 2016). Emerging studies have shown that among people with cancer, those who have received hematopoietic cell transplantation are at higher risk for diabetes following treatment (Clark, Savani, Mohty, & Savani, 2016; Fuji et al., 2016). Therefore, hyperglycemia must be accurately measured to be well managed. The ADA (2015) recommends that asymptomatic people who are overweight or obese or have other risk factors for diabetes be tested for diabetic status beginning at age 45 years. In addition, people who will be receiving glucocorticosteroids as part of their cancer treatment regimen should be routinely monitored for hyperglycemia and/or new onset of diabetes prior to the initiation of therapy, as well as throughout the regimen (Rowbottom et al., 2015).
Standard screening of people with cancer prior to the onset of treatment could be beneficial in predicting those most likely to experience hyperglycemia as well as assist in managing those patients who may be at a higher risk for development of hyperglycemia. Collaboration with an endocrinologist as part of the healthcare team is appropriate, particularly in situations in which blood glucose is unable to be maintained or adequately managed.
Implications for Nursing
The development of a standardized definition for and a process to measure hyperglycemia will facilitate its prevention and management, which may result in improving health-related outcomes in people with cancer. Nurses play a key role in the early identification of people experiencing hyperglycemia. As such, they can engage with and communicate the presence of hyperglycemia to oncology advanced practice nurses and physicians to initiate monitoring and treatment practices.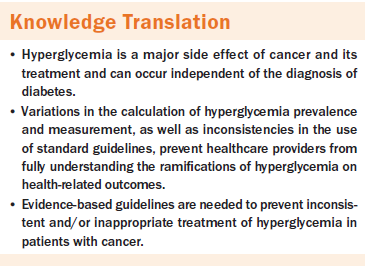
Conclusion
The need for evidence-based guidelines is imperative to prevent inconsistent and/or inappropriate treatment of hyperglycemia in patients with cancer. What has yet to be determined is whether the guidelines established by the ADA and/or critical care organizations are beneficial for patients with cancer, or if specific guidelines for treating people with cancer are needed. The harmful impact of hyperglycemia on the health outcomes of people with cancer cannot be fully appreciated until consistency exists among the definitions, thresholds, and measurement practices used to describe the phenomena. Early identification of people with cancer at higher risk for hyperglycemia can promote timely collaboration among the healthcare team to treat and mitigate the untoward effects of hyperglycemia. Future research should focus on defining hyperglycemia, identifying people with cancer at highest risk for hyperglycemia (including those with and without a diagnosis of diabetes), developing standards of practice and measurement, and creating recommendations for the use of technology in monitoring for hyperglycemia.
About the Author(s)
Storey is an assistant scientist and Von Ah is an associate professor, both in the School of Nursing at Indiana University in Indianapolis; and Hammer is the director of research and evidence-based practice in the Department of Nursing at Mount Sinai Hospital in New York, NY. No financial relationships to disclose. All authors contributed to the conceptualization and design, data collection, and manuscript preparation. Von Ah and Hammer provided statistical support and the analysis. Storey can be reached at sustorey@iu.edu, with copy to ONFEditor@ons.org. Submitted September 2016. Accepted for publication November 10, 2016.
References
Adham, S.A., Al Rawahi, H., Habib, S., Al Moundhri, M.S., Viloria-Petit, A., & Coomber, B.L. (2014). Modeling of hypo/hyperglycemia and their impact on breast cancer progression related molecules. PLOS ONE, 9, e113103. doi:10.1371/journal.pone.0113103
Ali, N.A., O’Brien, J.M., Jr., Blum, W., Byrd, J.C., Klisovic, R.B., Marcucci, G., . . . Grever, M.R. (2007). Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer, 110, 96–102. doi:10.1002/cncr.22777
American Diabetes Association. (2015). Introduction. Diabetes Care, 38(Suppl. 1), S1–S2. doi:10.2337/dc15-S001
American Diabetes Association. (2016). Classification and diagnosis of diabetes. Diabetes Care, 39(Suppl. 1), S13–S22. doi:10.2337/dc16-S005
Barba, M., Sperati, F., Stranges, S., Carlomagno, C., Nasti, G., Iaffaioli, V., . . . De Placido, S. (2012). Fasting glucose and treatment outcome in breast and colorectal cancer patients treated with targeted agents: Results from a historic cohort. Annals of Oncology, 23, 1838–1845. doi:10.1093/annonc/mdr540
Biernacka, K.M., Uzoh, C.C., Zeng, L., Persad, R.A., Bahl, A., Gillatt, D., . . . Holly, J.M. (2013). Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocrine-Related Cancer, 20, 741–751. doi:10.1530/ERC-13-0077
Brunello, A., Kapoor, R., & Extermann, M. (2011). Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. American Journal of Clinical Oncology, 34, 292–296. doi:10.1097/COC.0b013e3181e1d0c0
Cavalot, F., Petrelli, A., Traversa, M., Bonomo, K., Fiora, E., Conti, M., . . . Trovati, M. (2006). Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga Diabetes Study. Journal of Clinical Endocrinology and Metabolism, 91, 813–819. doi:10.1210/jc.2005-1005
Centers for Disease Control and Prevention. (2014). Diabetes report card 2014. Retrieved from http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf
Clark, C.A., Savani, M., Mohty, M., & Savani, B.N. (2016). What do we need to know about allogeneic hematopoietic stem cell transplant survivors? Bone Marrow Transplantation, 51, 1025–1031. doi:10.1038/bmt.2016.95
Danese, E., Montagnana, M., Nouvenne, A., & Lippi, G. (2015). Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. Journal of Diabetes Science and Technology, 9, 169–176. doi:10.1177/1932296814567227
Derr, R., Ye, X., Islas, M.U., Desideri, S., Saudek, C.D., & Grossman, S.A. (2009). Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. Journal of Clinical Oncology, 27, 1082–1086. doi:10.1200/JCO.2008.19.1098
Derr, R.L., Hsiao, V.C., & Saudek, C.D. (2008). Antecedent hyperglycemia is associated with an increased risk of neutropenic infections during bone marrow transplantation. Diabetes Care, 31, 1972–1977. doi:10.2337/dc08-0574
Dungan, K., Chapman, J., Braithwaite, S.S., & Buse, J. (2007). Glucose measurement: Confounding issues in setting targets for inpatient management. Diabetes Care, 30, 403–409. doi:10.2337/dc06-1679
Dungan, K.M., Braithwaite, S.S., & Preiser, J.-C. (2009). Stress hyperglycaemia. Lancet, 373, 1798–1807. doi:10.1016/S0140-6736(09)60553-5
Egi, M., Bellomo, R., Stachowski, E., French, C.J., Hart, G.K., Hegarty, C., & Bailey, M. (2008). Blood glucose concentration and outcome of critical illness: The impact of diabetes. Critical Care Medicine, 36, 2249–2255. doi:10.1097/CCM.0b013e318181039a
Falciglia, M. (2007). Causes and consequences of hyperglycemia in critical illness. Current Opinion in Clinical Nutrition and Metabolic Care, 10, 498–503. doi:10.1097/MCO.0b013e3281a3bf0a
Farmer, A.J. (2010). Monitoring diabetes. In R.I. Holt, C.S. Cockram, A. Flyvbjerg, & B.J. Goldstein (Eds.), Textbook of diabetes (4th ed., pp. 399–409). Hoboken, NJ: Blackwell Publishing.
Farrokhi, F., Smiley, D., & Umpierrez, G.E. (2011). Glycemic control in non-diabetic critically ill patients. Best Practice and Research. Clinical Endocrinology and Metabolism, 25, 813–824. doi:10.1016/j.beem.2011.05.004
Flood, A., Mai, V., Pfeiffer, R., Kahle, L., Remaley, A.T., Lanza, E., & Schatzkin, A. (2007). Elevated serum concentrations of insulin and glucose increase risk of recurrent colorectal adenomas. Gastroenterology, 133, 1423–1429. doi:10.1053/j.gastro.2007.08.040
Fuji, S., Kim, S.-W., Mori, S., Fukuda, T., Kamiya, S., Yamasaki, S., . . . Takaue, Y. (2007). Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation, 84, 814–820. doi:10.1097/01.tp.0000296482.50994.1c
Fuji, S., Kim, S.-W., Mori, S., Kamiya, S., Yoshimura, K., Yokoyama, H., . . . Fukuda, T. (2009). Intensive glucose control after allogeneic hematopoietic stem cell transplantation: A retrospective matched-cohort study. Bone Marrow Transplantation, 44, 105–111. doi:10.1038/bmt.2008.431
Fuji, S., Rovó, A., Ohashi, K., Griffith, M., Einsele, H., Kapp, M., . . . Savani, B.N. (2016). How do I manage hyperglycemia/post-transplant diabetes mellitus after allogeneic HSCT. Bone Marrow Transplantation, 51, 1041–1049. doi:10.1038/bmt.2016.81
Gallo, M., Gentile, L., Arvat, E., Bertetto, O., & Clemente, G. (2016). Diabetology and oncology meet in a network model: Union is strength. Acta Diabetologica, 53, 515–524. doi:10.1007/s00592-016-0839-z
Garg, R., Bhutani, H., Alyea, E., & Pendergrass, M. (2007). Hyperglycemia and length of stay in patients hospitalized for bone marrow transplantation. Diabetes Care, 30, 993–994. doi:10.2337/dc06-2563
Gebremedhin, E., Behrendt, C.E., Nakamura, R., Parker, P., & Salehian, B. (2013). Severe hyperglycemia immediately after allogeneic hematopoietic stem-cell transplantation is predictive of acute graft-versus-host disease. Inflammation, 36, 177–185. doi:10.1007/s10753-012-9533-7
Germenis, A.E., & Karanikas, V. (2007). Immunoepigenetics: The unseen side of cancer immunoediting. Immunology and Cell Biology, 85, 55–59. doi:10.1038/sj.icb.7100006
Hammer, M.J., Casper, C., Gooley, T.A., O’Donnell, P.V., Boeckh, M., & Hirsch, I.B. (2009). The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biology of Blood and Marrow Transplantation, 15, 344–351. doi:10.1016/j.bbmt.2008.12.488
Hammer, M.J., D’Eramo Melkus, G., Knobf, M.T., Casper, C., Fletcher, J., & Cleland, C.M. (2016). Glycemic status and infection risk in nondiabetic autologous hematopoietic cell transplantation recipients. Biological Research for Nursing, 18, 344–350. doi:10.1177/1099800415619227
Hardy, S.J., Nowacki, A.S., Bertin, M., & Weil, R.J. (2010). Absence of an association between glucose levels and surgical site infections in patients undergoing cranitomies for brain tumors. Journal of Neurosurgery, 113, 161–166. doi:10.3171/2010.2.JNS09950
Harris, D., Barts, A., Connors, J., Dahl, M., Elliott, T., Kong, J., . . . Sirrs, S. (2013). Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: An observational cohort study. Current Oncology, 20, e532–e538. doi:10.3747/co.20.1499
Hermanides, J., Vriesendorp, T.M., Bosman, R.J., Zandstra, D.F., Hoekstra, J.B., & DeVries, J.H. (2010). Glucose variability is associated with intensive care unit mortality. Critical Care Medicine, 38, 838–842. doi:10.1097/CCM.0b013e3181cc4be9
Hermayer, K.L., Loftley, A.S., Reddy, S., Narla, S.N., Epps, N.A., & Zhu, Y. (2015). Challenges of inpatient blood glucose monitoring: Standards, methods, and devices to measure blood glucose. Current Diabetes Reports, 15, 10. doi:10.1007/s11892-015-0582-9
Hershey, D.S., Bryant, A.L., Olausson, J., Davis, E.D., Brady, V.J., & Hammer, M. (2014). Hyperglycemic-inducing neoadjuvant agents used in treatment of solid tumors: A review of the literature [Online exclusive]. Oncology Nursing Forum, 41, E343–E354. doi:10.1188/14.ONF.E343-E354
Hirsch, I.B. (2010). How to proceed with glucose monitoring accuracy? An issue that requires immediate attention. Journal of Diabetes, 2, 225–226. doi:10.1111/j.1753-0407.2010.00095.x
Hong, Y.J., Han, H.-S., Jeong, Y., Jeong, J., Lim, S.-N., Choi, H.J., . . . Lee, K.H. (2014). Impact of hyperglycemia on survival and infection-related adverse events in patients with metastatic colorectal cancer who were receiving palliative chemotherapy. Cancer Research and Treatment, 46, 288–296. doi:10.4143/crt.2014.46.3.288
Ito, N., Iwaya, T., Ikeda, K., Kimura, Y., Akiyama, Y., Konosu, M., . . . Wakabayashi, G. (2014). Hyperglycemia 3 days after esophageal cancer surgery is associated with an increased risk of postoperative infection. Journal of Gastrointestinal Surgery, 18, 1547–1556. doi:10.1007/s11605-014-2587-0
Jackson, R.S., Amdur, R.L., White, J.C., & Macsata, R.A. (2012). Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. Journal of the American College of Surgeons, 214, 68–80. doi:10.1016/j.jamcollsurg.2011.09.016
Jiménez-Ibáñez, E.O., Castillejos-López, M., Hernández, A., Gorocica, P., & Alvarado-Vásquez, N. (2012). High mortality associated with hyperglycemia, neutrophilia, and lymphopenia in critically ill patients. Tohoku Journal of Experimental Medicine, 226, 213–220. doi:10.1620/tjem.226.213
Karnchanasorn, R., Malamug, L., Jin, R., Karanes, C., & Chiu, K. (2012). Association of hyperglycemia with prolonged hospital stay but no effect on engraftment after autologous hematopoietic stem cell transplantation. Endocrine Practice, 18, 508–518. doi:10.4158/EP11307.OR
Kellenberger, L.D., Bruin, J.E., Greenaway, J., Campbell, N.E., Moorehead, R.A., Holloway, A.C., & Petrik, J. (2010). The role of dysregulated glucose metabolism in epithelial ovarian cancer. Journal of Oncology, 2010, 514310. doi:10.1155/2010/514310
Luo, J., Chen, Y.-J., & Chang, L.-J. (2012). Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer, 76, 242–247. doi:10.1016/j.lungcan.2011.10.019
Malmström, H., Walldius, G., Grill, V., Jungner, I., Gudbjörnsdottir, S., & Hammar, N. (2014). Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies—Cross-sectional and longitudinal experience from the AMORIS cohort. PLOS ONE, 9, e111463. doi:10.1371/journal.pone.0111463
Masrur, S., Cox., M., Bhatt, D.L., Smith, E.E., Ellrodt, G., Fonarow, G.C., & Schwamm, L. (2015). Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thromoblysis: Findings from Get With the Guidelines–Stroke. Journal of the American Heart Association, 4, e002193. doi:10.1161/JAHA.115.002193
Matias, C.D.N., Lima, V., Teixeira, H.M., Souto, F.R., & Magalhães, V. (2013). Hyperglycemia increases the complicated infection and mortality rates during induction therapy in adult acute leukemia patients. Revista Brasileira de Hematologia e Hemoterapia, 35, 39–43. doi:10.5581/1516-8484.20130013
Mraovic, B., Hipszer, B.R., Epstein, R.H., Pequignot, E.C., Parvizi, J., & Joseph, J.I. (2010). Preadmission hyperglycemia is an independent risk factor for in-hospital symptomatic pulmonary embolism after major orthopedic surgery. Journal of Arthroplasty, 25, 64–70. doi:10.1016/j.arth.2008.10.002
Olausson, J.M., Hammer, M.J., & Brady, V. (2014). The impact of hyperglycemia on hematopoietic cell transplantation outcomes: An integrative review [Online exclusive]. Oncology Nursing Forum, 41, E302–E312. doi:10.1188/14.ONF.E302-E312
Oyer, D.S., Shah, A., & Bettenhausen, S. (2006). How to manage steroid diabetes in the patient with cancer. Journal of Supportive Oncology, 4, 479–483.
Pidala, J., Kim, J., Kharfan-Dabaja, M.A., Nishihori, T., Field, T., Perkins, J., . . . Anasetti, C. (2011). Dysglycemia following glucocorticoid therapy for acute graft-versus-host disease adversely affects transplantation outcomes. Biology of Blood and Marrow Transplantation, 17, 239–248. doi:10.1016/j.bbmt.2010.07.005
Rabkin, Z., Israel, O., & Keidar, Z. (2010). Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A comparative analysis. Journal of Nuclear Medicine, 51, 1015–1020. doi:10.2967/jnumed.109.074294
Rentschler, L.M., Swarts, S.J., Bierman, P.J., Devetten, M.P., Stoner, J.A., Puumala, S.E., & Goldner, W.S. (2010). Association between hyperglycemia and hospital length of stay in patients undergoing hematopoietic stem cell transplantation. Endocrinologist, 20, 232–235. doi:10.1097/TEN.0b013e3181f47dbc
Rowbottom, L., Stinson, J., McDonald, R., Emmenegger, U., Cheng, S., Lowe, J., . . . DeAngelis, C. (2015). Retrospective review of the incidence of monitoring blood glucose levels in patients receiving corticosteroids with systemic anticancer therapy. Annals of Palliative Medicine, 4, 70–77. doi:10.3978/j.issn.2224-5820.2015.04.07
Sarwar, N., Gao, P., Seshasai, S.R., Gobin, R., Kaptoge, S., Di Angelantonio, E., . . . Danesh, J. (2010). Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet, 375, 2215–2222. doi:10.1016/S0140-6736(10)60484-9
Schrot, R.J., Patel, K.T., & Foulis, P. (2007). Evaluation of inaccuracies in the measurement of glycemia in the laboratory, by glucose meters, and through measurement of hemoglobin A1c. Clinical Diabetes, 25, 43–49. doi:10.2337/diaclin.25.2.43
Sheean, P.M., Freels, S.A., Helton, W.S., & Braunschweig, C.A. (2006). Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation, 12, 656–664. doi:10.1016/j.bbmt.2006.01.010
Sheean, P.M., Kilkus, J.M., Liu, D., Maciejewski, J., & Braunschweig, C.A. (2013). Incident hyperglycemia, parenteral nutrition administration and adverse outcomes in patients with myeloma admitted for initial auto-SCT. Bone Marrow Transplantation, 48, 1117–1122. doi:10.1038/bmt.2013.11
Soysal, D.E., Karakus, V., Seren, A.R., Tatar, E., Celik, M., & Hizar, S. (2012). Evaluation of transient hyperglycemia in non-diabetic patients with febrile neutropenia. European Journal of Internal Medicine, 23, 342–346. doi:10.1016/j.ejim.2011.12.010
Storey, S., & Von Ah, D. (2012). Impact of malglycemia on clinical outcomes in hospitalized patients with cancer: A review of the literature. Oncology Nursing Forum, 39, 458–465. doi:10.1188/12.ONF.458-465
Storey, S., & Von Ah, D. (2015). Prevalence and impact of hyperglycemia on hospitalized leukemia patients. European Journal of Oncology Nursing, 19, 13–17. doi:10.1016/j.ejon.2014.08.005
Storey, S., & Von Ah, D. (2016). Impact of hyperglycemia and age on outcomes in patients with acute myeloid leukemia. Oncology Nursing Forum, 43, 595–601. doi:10.1188/16.ONF.595-601
Tieu, M.T., Lovblom, L.E., McNamara, M.G., Mason, W., Laperriere, N., Millar, B.-A., . . . Chung, C. (2015). Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. Journal of Neuro-Oncology, 124, 119–126. doi:10.1007/s11060-015-1815-0
Umpierrez, G.E., Isaacs, S.D., Bazargan, N., You, X., Thaler, L.M., & Kitabchi, A.E. (2002). Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. Journal of Clinical Endocrinology and Metabolism, 87, 978–982. doi:10.1210/jc.87.3.978
Villarreal-Garza, C., Shaw-Dulin, R., Lara-Medina, F., Bacon, L., Rivera, D., Urzua, L., . . . Herrera, L.A. (2012). Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Experimental Diabetes Research, 2012, 732027. doi:10.1155/2012/732027
Weiser, M.A., Cabanillas, M.E., Konopleva, M., Thomas, D.A., Pierce, S.A., Escalante, C.P., . . . O’Brien, S.M. (2004). Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer, 100, 1179–1185. doi:10.1002/cncr.20071
Whittemore, R., & Knafl, K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52, 546–553. doi:10.1111/j.1365-2648.2005.03621.x
Wright, J.L., Plymate, S.R., Porter, M.P., Gore, J.L., Lin, D.W., Hu, E., & Zeliadt, S.B. (2013). Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer and Prostatic Diseases, 16, 204–208. doi:10.1038/pcan.2013.5
Zuurbier, S.M., Hiltunen, S., Tatlisumak, T., Peters, G.M., Silvis, S.M., Haapaniemi, E., . . . Coutinho, J.M. (2016). Admission hyperglycemia and clinical outcomes in cerebral venous thrombosis. Stroke, 47, 390–396. doi:10.1161/STROKEAHA.115.011177

