Ethical Considerations for Data Collection Using Surveys
Surveys are widely used instruments to collect research data. Although surveys may appear relatively benign and easily unlinked to participants, considerations for the ethical conduct of research with surveys are important. Maintaining scientific rigor is essential. This article explores ethical tenets in relation to informed consent and scientific consent when using surveys.
Jump to a section
Surveys are instruments used to quantitatively evaluate subjective data. Through the addition of open-ended questions, qualitative data can also be obtained (Eysenbach & Wyatt, 2002). Technically, survey is a general term used to describe the collection of information but is often used interchangeably with questionnaire—a list of focused questions. In oncology, assessing symptoms and quality of life (QOL) through surveys aids in providing feedback to optimize patient care. The collecting and analyzing of patient information is integral to research and patient care. One of the National Institutes of Health’s initiatives within the Roadmap for Medical Research was the development of the Patient-Reported Outcomes Measurement Information Systems (PROMIS®) (Ader, 2007). Domains within PROMIS specifically target symptoms and QOL issues of patients with cancer (Garcia et al., 2007). Aside from PROMIS, numerous valid and reliable instruments are used to gather patient experiences and needs in oncology. These instruments have also been paired with biomarkers to investigate associations between patient perceived experiences and underlying mechanisms. For example, the research program conducted by Miaskowski (2016) and others (Alfaro et al., 2014; Dhruva et al., 2014; Merriman et al., 2014) highlights genetic variants and symptom clusters.
Although there can be multiple variations of simple surveys and complex questionnaires paired with biomarker data, research ethics should be universally maintained. When using surveys, two areas to consider are obtaining informed consent and maintaining scientific integrity.
Obtaining Informed Consent
Obtaining informed consent may vary in format. Often, in-person participant enrollment in studies includes a discussion and signing a consent form. Mailed or web-based surveys, however, have implied consent or “passive” consent by virtue of participants completing them (Buchanan & Hvizdak, 2009). However the survey is delivered, participants should understand that they have the right to participate without compromise of care. In addition, they should have the right to not answer specific questions. This is not an issue when using paper surveys, but sometimes electronic surveys do not allow participants to skip questions. Participants may not be allowed to move on to a subsequent question without responding to the current question, or, in some cases, they may not be able to submit a survey without responding to all questions. If answers are not allowed to be skipped, participants may not complete a survey or may provide false information that is not representative of their specific situations. Aside from actual content, surveys should not require answers to demographic questions. Missing data can be statistically adjusted for, allowing participants to skip questions. Alternatively, surveys with missing data can be eliminated from the analyses, as seen in a study that evaluated pragmatic randomized trials without standard informed consent (Nayak, Wendler, Miller, & Kim, 2015).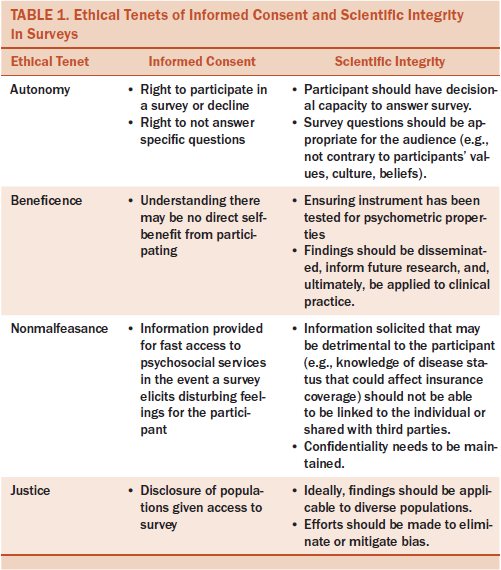
Other considerations include participants’ understanding the direct benefit of a survey, which may not exist, and that some questions can trigger disturbing and unfavorable feelings or memories. If participants are completing surveys in person, this can readily be addressed by immediate referral to psychosocial services. It is more difficult to track participant reactions to mailed or web-based surveys; however, referral contact information can be provided at the beginning and/or end of a survey. When evaluating proposals that include surveys, institutional review boards may look for a plan to address psychological distress elicited from questions (Eysenbach & Wyatt, 2002). Researchers should also disclose the target population of the survey and why it was chosen in the consent form or as a statement prior to delivering mailed or web-based surveys. Based on this information, participants should be able to choose whether to participate.
Maintaining Scientific Integrity
Maintaining scientific integrity when using surveys, whether in-person, mailed, or web-based, is important. First, participants should have the decisional capacity to answer surveys, which can be difficultfor researchers to determine if the survey is mailed or web-based. Researchers should also ensure that the survey questions are appropriate for the audience (i.e., not contrary to participants’ values, culture, or beliefs) (Kraft et al., 2016). Also of high importance is using psychometrically tested surveys. Any individual can create and post a web-based survey, so the integrity, validity, and reliability of survey questions can be questionable without such an evaluation. The PROMIS questionnaires are electronically based and boast strong psychometric properties (Ader, 2007). Some established paper surveys have also been evaluated for the maintenance of validity and reliability when converted to electronic formats. For example, a health-related QOL and patient-reported outcomes questionnaire was tested in both paper and electronic versions in a sample of patients with non-small cell lung cancer. Results indicated that the electronic version was highly acceptable and maintained the existing validity and reliability of the paper version (Hollen et al., 2012).
Protecting privacy is another concern when using surveys (Eysenbach & Wyatt, 2002). Participants completing electronic or web-based surveys may seem completely anonymous; however, individual internet protocol (IP) addresses can be traced (Baker, 2012). Researchers may identify IP addresses to ensure that duplicate responses from the same subject are not included in the dataset (Baker, 2012). Surveys can also be programmed to not allow duplicate responses from the same IP address. As with any research study, connecting responses to participants with health issues can potentially affect their health insurance coverage and employment.
In all forms of research, minimizing bias is important. Selection bias can be problematic and can also compromise external validity in posted web-based surveys (Eysenbach & Wyatt, 2002). In addition, researchers should attempt to target diverse populations when it does not interfere with the research purpose. Finally, research findings should be disseminated. Using participants’ time to fill out surveys is just as important as involving them in other types of research, such as testing for biomarkers or an intervention. Publishing and/or presenting the findings at meetings and conferences and, ultimately, translating the findings to patient practice are not only respectful to participants but moves the science forward toward improved understanding and patient care. Table 1 outlines the ethical tenets of informed consent and scientific integrity when using surveys.
Conclusion
Research surveys can be very informative. Even when surveys seem innocuous, ensuring that the tenets of the ethical conduct of research are maintained is paramount. Maintaining fidelity and scientific rigor is important in all research studies. Using valid and reliable survey instruments, applying proper analytical methods, and disseminating finding are essential. Finally, disclosing the format in which surveys are distributed, how consent is obtained or determined exempt, and how the data are monitored is important.
About the Author(s)
Hammer is the director of research and evidence-based practice in the Department of Nursing at Mount Sinai Hospital in New York, NY. No financial relationships to disclose. Hammer can be reached at marilyn.hammer@mountsinai.org, with copy to editor at ONFEditor@ons.org.

