Equivalence Study of Two Temperature-Measurement Methods in Febrile Adult Patients With Cancer
Purpose/Objectives: To assess equivalence of temperatures taken via temporal artery and oral methods in febrile inpatients with cancer.
Design: Repeated measures equivalence design.
Setting: 24-bed hematology-oncology unit and 36-bed blood and marrow transplantation unit at H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute–designated comprehensive cancer center in Tampa, Florida.
Sample: Convenience sample of 58 febrile inpatients using 60 temperature measurements.
Methods: Two instruments were used: Welch Allyn SureTemp® Plus 690 (oral) and Exergen TAT-5000 (temporal artery). Temperatures were measured closely following instrument instructions by three highly experienced nurses.
Main Research Variables: Temperature readings from oral and temporal artery measurements.
Findings: A two one-sided test (TOST) technique with a delta of 0.2ºF was performed to assess equivalence of the oral and temporal artery measures. Within each team member, the oral and temporal artery measures were assessed using the TOST technique. The hypothesis of equivalence was rejected for all three team members.
Conclusions: The use of the Exergen TAT-5000 for temperature measurement as a noninvasive alternate to the oral method for febrile adult patients in the hematology-oncology population was not supported.
Implications for Nursing: The Exergen TAT-5000 is not a reliable instrument for detecting fevers in the hematology-oncology patient population because the temporal artery measures were not equivalent to oral measures, and considerable variation occurred for the three nurses. Alternative methods for accurate temperature collection need to be investigated, particularly for febrile, neutropenic patients who cannot tolerate the oral probe.
Jump to a section
Fever in immunocompromised patients, particularly those with neutropenia, is widely recognized as an oncologic emergency that may indicate sepsis or impending septic shock. In the event of sepsis, the initial change in vital signs is often temperature elevation greater than or equal to 100.5°F. A new onset of fever will activate the need for clinical decisions, initiation of a neutropenic fever protocol, and other potentially lifesaving measures. Early recognition of fever in the oncology population depends on accurate and precise monitoring of body temperature. Some methods of body temperature measurement are problematic in immunocompromised patients.
Background
Historically, methods of body temperature measurement used throughout inpatient clinical areas included oral, axillary, tympanic, and rectal. The oral temperature is the recommended noninvasive measure to monitor temperature when invasive methods to obtain the core body temperature are not feasible or practical. When use of the oral probe is not possible, it is common practice to use an axillary probe. Factors that preclude the use of oral, axillary, and rectal devices with neutropenic patients are encountered frequently when caring for them. Newer methods are associated with devices that measure temperature using the temporal artery.
Neutropenic patients are often unable to tolerate oral probe placement. Changes in the oral mucosa, such as with mucositis or xerostomia, can lead to pain during the probe placement and may result in incomplete closure of the mouth or an inability to keep the probe seated in the sublingual pocket. Inaccuracy in temperature measurement is the consequence. Difficulty with probe placement may also occur during rigor with acute onset of fever. Oral inflammatory processes, such as mucositis, may cause false elevations in temperature when using an oral probe thermometer (Bridges & Thomas, 2009). Recent ingestion of cold or hot liquids, recent smoking, gum chewing, or high-flow oxygen therapy via mask or nasal cannula flowing at greater than 6 L have all been noted to cause inaccuracies in temperature monitoring (Quatrara et al., 2007).
The axillary thermometer probe needs to be completely surrounded by tissue (Bridges & Thomas, 2009). Limiting factors in accuracy include diaphoresis and cachexia, resulting in improper seating of the probe in the axilla. Axillary temperatures were also deemed inaccurate because of alteration in skin integrity or vasoconstriction (Hutton et al., 2009; Mangat, Standley, Prevost, Vasconcelos, & White, 2010; Myny, De Waele, Defloor, Blot, & Colardyn, 2005).
Researchers have reported that inaccurate measures obtained with the tympanic monitors were because of user error and common alterations in the ear canal (e.g., debris in the canal) (Lawson et al., 2007). Axillary temperatures were found to underestimate body temperature, and tympanic thermometers were found to be least accurate and precise (Lawson et al., 2007). Rectal temperatures should be avoided in patients with neutropenia because of potential risk of infection, as well as thrombocytopenia, which can be a risk for bleeding (Polovich, Olsen, & LeFebvre, 2014).
Numerous studies have examined the use of the temporal artery thermometer (TAT). Lawson et al. (2007) compared alternative methods of temperature measurement using pulmonary artery catheter thermometers in adult patients in intensive care. Oral and temporal artery measurements were found to be the most accurate and precise compared with core temperatures. Other studies found favorable results using a TAT developed by the Exergen Corporation. No significant difference between the pulmonary artery reading and the Exergen Model LXTA use for a temporal artery measurement taken from the forehead and behind the ear was found by Carroll, Finn, Judge, Gill, and Sawyer (2004). In a study comparing oral and temporal artery (Exergen TAT-5000) compared to core via esophageal probe, oral and temporal artery thermometry overestimated the esophageal temperature, with temporal artery being more accurate than oral (Calonder et al., 2010). Myny et al. (2005) compared the Exergen Model LXTA with axillary and pulmonary artery readings. Mean difference of the TAT was relatively good compared to pulmonary artery catheter temperature readings. Barringer et al. (2011) examined agreement in pre- and postoperative temperature readings measured with a TAT and electronic oral or axillary artery thermometers. Results supported using the TAT as an alternative in normothermic, adult surgical patients. Inter-rater reliability was not reported.
The accuracy and reliability of the TAT (Calonder et al., 2010; Carroll et al., 2004; Myny et al., 2005) has been questioned. TAT devices are known to differ with the technique used. Devices that combine a forehead sweep with a behind-the-ear scan have been shown to have increased reliability. Airflow across the face (causing a change in ambient temperature), variability of skin thickness, and diaphoresis were offered as potential variables that may affect TAT readings (Bridges & Thomas, 2009; Lawson et al., 2007; Mangat et al., 2010; Myny et al., 2005; Suleman, Doufas, Akca, Ducharme, & Sessler, 2002). Several studies concluded that the TAT is less reliable in febrile patients (Fountain et al., 2008; Lawson et al., 2007; Low et al., 2007; Mangat et al., 2010). In a review of the literature, Bahr, Senica, Gingras, and Ryan (2010) noted that existing research did not provide adequate evidence to support the use of TAT for acutely ill hospitalized patients. Limitations of studies include that some may be biased because of a collaborative relationship with the TAT developers, and many had small sample sizes and did not report a power analysis (Bahr et al., 2010).
In study by Mason et al. (2015), a repeated measures equivalence design was used to test temperatures taken with three noninvasive devices: standard electronic thermometer in oral mode (O), standard electronic thermometer in axillary mode (A), and Exergen TAT-5000 (T). To control for possible carry-over effects, a Latin square design was employed with three possible sequences of measurement (OAT, ATO, TOA). Prior to study initiation on an adult hematology-oncology unit, inter-rater reliability (Cohen’s kappa > 0.8) was achieved by the two data collectors. Equivalence was demonstrated for temporal artery and oral measurements in degrees Fahrenheit (90% confidence interval [CI] [0.14, 98.38]) but not for axillary and oral measurements (90% CI [0.25, 98.49]). Findings suggested that axillary measurements should be used with caution or discontinued. The study supported use of the Exergen TAT-5000 for temperature measurement in the afebrile hematology-oncology patient population. Because all patients were afebrile and researchers in other studies have reported lower levels of reliability when using the Exergen TAT-5000 with febrile patients, the current follow-up study was conducted using a sample of febrile patients.
Obtaining accurate temperature in the oncology setting is vital. An alternative to the oral thermometer is needed when a core temperature cannot be obtained. Although the TAT has demonstrated equivalence in studies, it has been questioned for use in febrile patients. The aim of the current study was to determine equivalence of temperatures taken via temporal artery and oral methods and determine if the temporal artery method works equally as well as the oral method in the febrile adult patients with cancer.
Methods
A repeated measures equivalence design was used to compare temperatures in adult febrile patients with cancer using two noninvasive methods for comparison: a standard electronic thermometer in oral mode and a TAT. When a patient was identified as febrile and met inclusion criteria, an oral temperature was retaken with the study device followed by the temporal scan as a comparison. The study was conducted on a 24-bed hematology-oncology unit and a 36-bed blood and marrow transplantation unit (BMTU) at H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute–designated comprehensive cancer center in Tampa, Florida.
Sample
A convenience sample of 58 inpatients was tested using 60 temperature measurements. Inclusion criteria were (a) being admitted to the hematology-oncology unit or BMTU, (b) having a temperature of 100.5°F (38°C) or greater per measurement with an oral probe, (c) being aged 18 years or older, (d) being able to understand directions of the researcher collecting data, (e) having the ability to maintain supine or sitting positions during data collection, and (f) being able to have the oral probe placed in the deep sublingual pocket. Exclusion criteria were (a) mucositis greater than grade 2 per the National Cancer Institute Common Terminology Criteria for Adverse Events (U.S. Department of Health and Human Services, 2010); (b) oxygen therapy greater than 6 L via nasal cannula or the use of a face mask; (c) isolation (e.g., contact, respiratory); and (d) any skin irritation or inflammation because of graft-versus-host disease, infectious process, rash, or leukemia cutis in the area where the TAT probe would be used.
The mean and standard deviation of oral temperatures of patients on the hematology-oncology unit and BMTU were computed from data documented routinely in the electronic health record (EHR). These data were used in an a priori power analysis and sample size calculation for the study. Most participants had one set of measurements taken for the study; however, if the patient was febrile more than 12 hours later, a second set of measurements could be taken.
Instruments
The instruments used were (a) the Welch Allyn (2015) SureTemp® Plus 690, a portable thermometer used for body temperature measurement at oral, axillary, or rectal sites for adult and pediatric populations and the standard of care practice and (b) the Exergen TAT-5000, a thermometer that measures body temperature via temporal artery through the patient’s skin at the forehead and the temporal site of the patient’s head (Exergen Corporation, 2010). The Exergen TAT-5000 is known for its antibacterial head; continuous ions are released from its silver-impregnated head that lasts for five years and provides contact coverage against a broad spectrum of bacteria, fungi, and other microorganisms. Two sets of instruments were used. One set was shared by two data collectors on BMTU, and one set was used by the data collector on the hematology-oncology unit. Thermometers were calibrated by the Biomedical Asset Management Department of the institution. Data collectors’ training included review of all manufacturers’ education materials for the Exergen TAT-5000 (Exergen Corporation, 2007) and demonstration of temperature measurement using the Exergen TAT-5000 on healthy volunteers. The demonstration was monitored by clinical nurse specialists. Neither manufacturer of these devices was involved in this study.
Data Collection
Nurses and oncology technicians were reminded daily to communicate to the available data collector whether any patient had an oral temperature of 100.5ºF (38ºC) or greater. When a patient was identified, the EHR was reviewed and inclusion and exclusion criteria were verified. The data collector consulted with the RN caring for the patient for an appropriate time to approach the patient. At the bedside, the data collector explained the study to the patient and that the temperature would be taken by two different thermometers. The data collector confirmed with the patient that no cold or hot food or fluids had been ingested by mouth for the past 20 minutes; if they had, 20 minutes were allowed to pass before taking temperatures. Data were collected and logged on the approved form and secured in a specific locked cabinet with the study instruments.
Ethical Considerations
Regulatory approval was obtained from the H. Lee Moffitt Cancer Center and Research Institute scientific review committee and the Liberty Institutional Review Board in Deland, Florida. Informed consent was waived for this study because the methods of temperature measurement (oral and temporal artery) are noninvasive and used for routine care provided to patients. No increased risk or burden was placed on the participant, and participation was voluntary.
Results
Each data collector assessed temperatures from 20 different patients. A two one-sided test (TOST) technique with a delta of 0.2ºF was performed to assess equivalence of the oral and temporal measures. Across all 60 observations, the average oral–temporal artery difference was 0.137 (90% CI [0.18, 0.453]). Based on this upper limit value, the hypothesis of equivalence with a delta of 0.2ºF was rejected.
[[{"type":"media","view_mode":"media_original","fid":"30116","attributes":{"alt":"","class":"media-image","height":"489","typeof":"foaf:Image","width":"372"}}]]
Figures 1–3 present scatterplots of the oral and temporal artery temperatures for each of the three data collectors. Each panel presents the 20 data points for one data collector, with oral on the x-axis and temporal artery on the y-axis. The solid line represents the least-squares regression slope for that data collector. The less scatter of the data points around the line, the greater the equivalence. The dashed line (slope = 1) represents ideal measurement and correspondence of the two instruments. Regression slopes that deviate from 1 suggest a bias in the use of one or both instruments.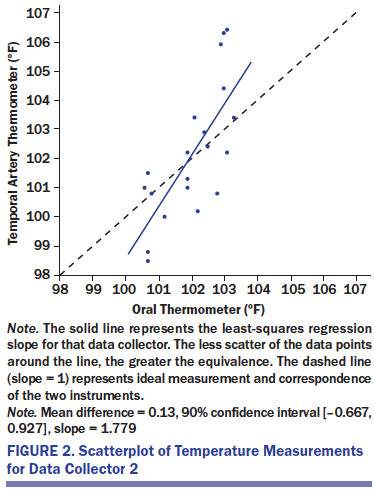
The oral and temporal artery measures were assessed using the TOST technique for each data collector. The hypothesis of equivalence was rejected for all three data collectors. This is highlighted in the figure by the dispersion of data points around the regression line. In addition, only one of the three regression lines has a slope close to 1 (collector 3), which represents a perfect correspondence of the two measures. Collector 1 has a more restricted range of temporal artery than oral temperatures, and collector 2 has a more restricted range of oral than temporal artery temperatures.
[[{"type":"media","view_mode":"media_original","fid":"30121","attributes":{"alt":"","class":"media-image","height":"494","typeof":"foaf:Image","width":"369"}}]]
Discussion
Study findings do not support the use of the Exergen TAT-5000 for temperature measurement as a noninvasive alternative to the oral method for febrile, adult patients in the hematology-oncology population. Although a previous study by the first author of the current study (Mason et al., 2015) found equivalence between oral temperature measurements and the Exergen TAT-5000, the patients in that study were afebrile.
In this study, all patients were febrile, and equivalence with the oral febrile temperature reading was not demonstrated with the Exergen TAT-5000. If the Exergen TAT-5000 was the only instrument used to measure temperature, seven neutropenic fevers potentially would have been missed. Therefore, the recommendation is that the Exergen TAT-5000 should not be used in the oncology patient population, for whom early identification of neutropenic fever is imperative. At the study site, the use of the Exergen TAT-5000 has been discontinued.
The current study is limited by the use of a convenience sample of febrile patients. They were drawn from two similar hematology-oncology inpatient units.
The results of this study suggest that the Exergen TAT-5000 is not a viable alternative to oral temperature measurement in febrile patients with cancer. Based on the current study, the Exergen TAT-5000 should be used with caution with patients in hematology-oncology units, where early identification of neutropenic fever is imperative.
Implications for Nursing
The TAT used in this study was not a reliable instrument for detecting fevers in the hematology-oncology patient population. Results obtained with the TAT were variable, sometimes reading higher than the oral thermometer and sometimes reading lower. Despite extensive training of the data collectors, each obtained variable results and compared the results to the other two users. Alternative methods for accurate temperature collection need to be investigated, particularly for neutropenic patients who cannot tolerate the oral probe.
Conclusion
A past study by the first author established equivalence between oral temperature measurement and temporal artery temperature measurement using the Exergen TAT-5000 in nonfebrile adult patients with cancer (Mason et al., 2015). The current study sought to establish the same equivalence in febrile adult patients with cancer. All data collectors had extensive training, and the TAT was calibrated by the biomedical engineering department prior to study commencement. The calibrated TATs were used only for the study. Despite these efforts, the study team was not able to establish equivalence between oral and temporal artery temperature measurement. In addition, if relying only on the TAT, seven neutropenic fevers might have been missed.
The authors gratefully acknowledge the patient care managers, biomedical asset management, Department Chair of Hematologic Malignancies, and other nurses on the inpatient unit for their support of this study.
About the Author(s)
Mason is an oncology clinical specialist in the Nursing Professional Department, Boubekri is a nurse practitioner in Internal and Hospital Medicine, Lalau is a staff RN III in the Clinical Research Unit, Patterson is a blood and marrow transplantation clinical specialist in the Nursing Professional Department, Hartranft is the director of nursing research in the Nursing Research Department, and Sutton is an assistant member in the Department of Biostatistics and Bioinformatics, all at the H. Lee Moffitt Cancer Center and Research Institute in Tampa, FL. No financial relationships to disclose. Mason, Lalau, Hartranft, and Sutton contributed to the conceptualization and design. Boubekri and Lalau completed the data collection. Sutton provided statistical support. All of the authors provided the analysis and contributed to the manuscript preparation. Mason can be reached at tina.mason@moffitt.org, with copy to editor at ONFEditor@ons.org. Submitted April 2016. Accepted for publication June 6, 2016.

