The Three-Generation Pedigree: A Critical Tool in Cancer Genetics Care
Pedigree construction is an important component of cancer risk assessment and comprehensive genetic care. Pedigrees must be updated and reevaluated on a regular basis. Complete pedigrees are needed to select genetic tests and interpret genetic testing results accurately, as well as to enroll patients and families in research and variant reclassification studies to advance the science of genetics. Identified barriers to pedigree construction and assessment are described with implications for nursing practice.
Jump to a section
The family history, a rather low-tech tool, is the backbone of genetic assessment and guides risk assessment and genetic testing decisions. The importance of the pedigree and its application to genetic practice is often overlooked and underestimated. Unfortunately, particularly with electronic health records, standard pedigrees are not routinely constructed. A clear understanding of how pedigrees are employed in clinical oncology practice may lead to improved collection and use of family history data.
Standardized Pedigree Nomenclature
Family histories of disease have been recorded in standardized formats for centuries (Resta, 1993). In ancient Greece, the physician Hippocrates assembled family histories to understand disease prognoses (Hinton, 2008). In 1995, standardized criteria for the graphic presentation of data were published by the National Society of Genetic Counselors (NSGC) (Bennett, French, Resta, & Doyle, 2008). Pedigrees can be constructed with pencil and paper and scanned into the record. More commonly, a pedigree software program is used to construct the pedigree, making the process of updating it as more pertinent information becomes available an easier task.
American Society of Clinical Oncology and NSGC guidelines emphasize that the family history should be obtained and evaluated at the initial visit and should be reevaluated at least annually (Berliner, Fay, Cummings, Burnett, & Tillmanns, 2013; Lu et al., 2014). A pedigree should include three generations, ancestry from the maternal and paternal sides, current age and age at death, and, in oncology, information about malignancies and age of diagnosis.
Clinical Applications
Like many aspects of health care, collecting an accurate family history is a science and an art (Venne & Scheuner, 2015). Obtaining a family history provides a unique opportunity to connect with the patient on a different level and learn more about family dynamics and social norms, providing insight as to how the family may manage information gleaned from risk assessment and genetic testing. If patients know prior to their appointment what information is required from them, they can then provide a more accurate family history and will have greater confidence in their risk assessment and screening recommendations (Armel et al., 2009). This also communicates the importance of the family history in clinical decision making. Despite these benefits, in addition to others that include gathering critical information about potential cancer and genetic risk to guide recommendations, constructing a complete three-generation pedigree takes time, often as long as 10–15 minutes (Mahon, 2013).
Ongoing assessment and pedigree updates are important because family histories are dynamic. The pedigree should be updated on a regular (annual) basis or as more information becomes available (Berliner et al., 2013). A common scenario often detected on reevaluation is that another relative is diagnosed with cancer and that the family history may be suggestive of genetic risk, necessitating genetic evaluation. Reevaluation is also important because more testing may have become available in the interim since the last evaluation. In addition, some patients with cancer may be more motivated and able to learn more about their family history once they have shifted beyond the stress of their initial diagnosis and focused on providing useful information for other at-risk family members (Venne & Scheuner, 2015).
Pedigrees provide a wide range of information. The proband is the person for whom the risk is being calculated and who provides orientation for the pedigree regarding how relatives are related and possibly share the same risk of disease. Cancer risk assessment depends on the construction of a pedigree. The more accurate and complete the pedigree is, the more accurate the assessment of the risk of developing cancer or the risk of having a susceptibility mutation (see Figure 1). 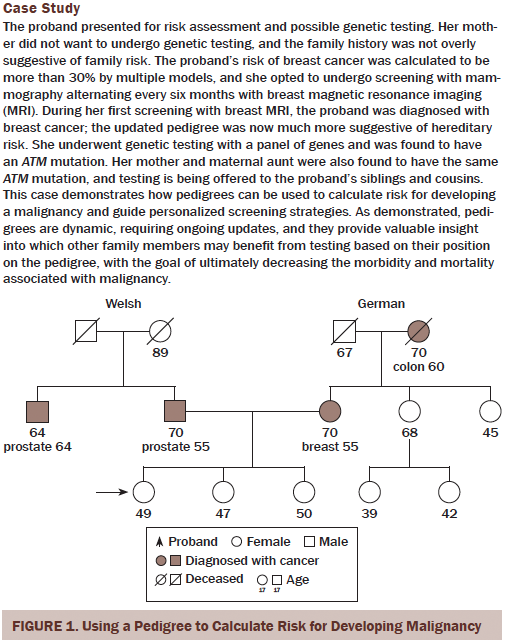
With the advent of next-generation testing for less penetrant and common genes, more variants of unknown clinical significance are detected (Mahon, 2015). To move forward and reclassify a variant of unknown significance, genetic testing laboratories will often want to test other family members who might provide valuable information about the variant based on their position in the pedigree. A standard pedigree is necessary for families who want to enroll in variant reclassification studies or studies that seek to identify less common susceptibility genes (see Figure 2).
Genetic testing results cannot be interpreted without an accurate and complete pedigree. Recommendations for care should include not only the results of testing, but also consideration of the family history of malignancy. This can be the case in positive and noninformative testing results.
Barriers and Limitations in Pedigree Assessment
Unfortunately, family histories are not always collected or are collected in such a way that they do not provide enough information to quantify risk. The visual format of the pedigree helps the healthcare professional better recognize potential hereditary susceptibility. Without the visual representation, potential risk may be overlooked. An audit of 10,466 charts from 212 oncology practices found that the provider documented the presence or absence of malignancy in first-degree relatives in about 77% of all medical records and in 62% for second-degree relatives (Wood et al., 2014). In addition, the age of diagnosis, which is an important component in evaluating risk, was only present in about 31% of the records.
Barriers hinder implementation of the pedigree. Family communication and dynamics may limit the availability and the accuracy of the information (see Figure 3). Healthcare providers may not have the time to accurately collect and document a pedigree, and they may not have the tools to create a meaningful and complete three-generation pedigree. Patient, provider, and system barriers are listed and described in Table 1.
Implications for Nurses
The three-generation pedigree is the key to calculating risk for developing cancer. Individuals whose pedigree suggests higher risk may benefit from modified guidelines for cancer prevention. The pedigree also provides the best means for identifying individuals and families who may benefit from genetic testing. Failure to identify at-risk families can result in less-than-adequate care and sometimes litigation (Robson et al., 2015).
The family history is a powerful tool for education and empowerment (Hinton, 2008) that should be shared with the patient (Lu et al., 2014). For many patients, the visual presentation of the family history helps them to understand the magnitude of the risk and can be a motivator to engage in the recommended prevention and detection measures (Beadles et al., 2014).
[[{"type":"media","view_mode":"media_original","fid":"25556","attributes":{"alt":"","class":"media-image","height":"695","typeof":"foaf:Image","width":"758"}}]]
A need exists for tools that can assist patients and families with collecting information about family history. One such tool is the Surgeon General’s My Family Health Portrait website (https://familyhistory.hhs.gov/FHH/html/index.html), which allows a family to store and share information about its history, as well as learn about its risk for genetic conditions (Owens, Marvin, Gelehrter, Ruffin, & Uhlmann, 2011). Nurses need to encourage patients to accurately collect as much information as possible. Patients and families need to understand that the more accurate the information, the better the recommendations for genetic testing, prevention, and detection. The importance of a complete and accurate three-generation pedigree should not be underestimated.
Conclusion
Standard pedigrees provide a visual representation of a family history of cancer. They are dynamic and must be updated as family history changes or as more is learned about the family history of malignancy. In addition, pedigrees provide an excellent teaching tool to demonstrate basic concepts of genetic transmission. Pedigrees also provide the backbone for cancer risk assessment, and the accuracy and completeness with which they are constructed can ultimately lead to better recommendations for cancer prevention and early detection, with the goal of decreasing the morbidity and mortality associated with malignancy.
Nurses need to be aware of the multiple ways that an accurate and well-constructed pedigree can influence patient care. For example, pedigrees can be used to calculate the risks of malignancy; when risks are elevated (typically greater than 20%), screening recommendations can be modified from those used with the general public of average risk. Pedigrees provide information about the transmission of disease and can also offer information about which family member would be best to initiate genetic testing, with the objective of obtaining illuminating information about genetic risk. If a mutation is detected, the pedigree can be used to identify other family members at possible increased risk; these individuals should then be offered the option of testing. Accurate and well-constructed pedigrees also advance the science of genetics and are necessary when enrolling families in variant reclassification studies, as well as studies intended to identify new genes associated with cancer risk. Nurses need to instruct patients about these numerous clinically important uses of the pedigree to engage them to provide the most accurate information possible.
References
Armel, S.R., McCuaig, J., Finch, A., Demsky, R., Panzarella, T., Murphy, J., & Rosen, B. (2009). The effectiveness of family history questionnaires in cancer genetic counseling. Journal of Genetic Counseling, 18, 366–378. doi:10.1007/s10897-009-9228-x
Beadles, C.A., Ryanne Wu, R., Himmel, T., Buchanan, A.H., Powell, K.P., Hauser, E., . . . Orlando, L.A. (2014). Providing patient education: Impact on quantity and quality of family health history collection. Familial Cancer, 13, 325–332. doi:10.1007/s10689-014-9701-z
Bennett, R.L., French, K.S., Resta, R.G., & Doyle, D.L. (2008). Standardized human pedigree nomenclature: Update and assessment of the recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 17, 424–433. doi:10.1007/s10897-008-9169-9
Berliner, J.L., Fay, A.M., Cummings, S.A., Burnett, B., & Tillmanns, T. (2013). NSGC practice guideline: Risk assessment and genetic counseling for hereditary breast and ovarian cancer. Journal of Genetic Counseling, 22, 155–163. doi:10.1007/s10897-012-9547-1
Hinton, R.B., Jr. (2008). The family history: Reemergence of an established tool. Critical Care Nursing Clinics of North America, 20, 149–158. doi:10.1016/j.ccell .2008.01.004
Lu, K.H., Wood, M.E., Daniels, M., Burke, C., Ford, J., Kauff, N.D., . . . Hughes, K.S. (2014). American Society of Clinical Oncology expert statement: Collection and use of a cancer family history for oncology providers. Journal of Clinical Oncology, 32, 833–840. doi:10.1200/jco.2013.50.9257
Mahon, S.M. (2013). Allocation of work activities in a comprehensive cancer genetics program. Clinical Journal of Oncology Nursing, 17, 397–404. doi:10.1188/13.CJON.397-404
Mahon, S.M. (2015). Management of patients with a genetic variant of unknown significance. Oncology Nursing Forum, 42, 316–318. doi:10.1188/15.ONF.316-318
Owens, K.M., Marvin, M.L., Gelehrter, T.D., Ruffin, M.T., IV, & Uhlmann, W.R. (2011). Clinical use of the Surgeon General’s “My Family Health Portrait” (MFHP) tool: Opinions of future health care providers. Journal of Genetic Counseling, 20, 510–525. doi:10.1007/s10897-011-9381-x
Resta, R.G. (1993). The crane’s foot: The rise of the pedigree in human genetics. Journal of Genetic Counseling, 2, 235–260. doi:10.1007/bf00961574
Riley, B.D., Culver, J.O., Skrzynia, C., Senter, L.A., Peters, J.A., Costalas, J.W., . . . Trepanier, A.M. (2012). Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors. Journal of Genetic Counseling, 21, 151–161. doi:10.1007/s10897-011-9462-x
Robson, M.E., Bradbury, A.R., Arun, B., Domchek, S.M., Ford, J.M., Hampel, H.L., . . . Lindor, N.M. (2015). American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. Journal of Clinical Oncology, 33, 3660–3667. doi:10.1200/jco .2015.63.0996
Venne, V.L., & Scheuner, M.T. (2015). Securing and documenting cancer family history in the age of the electronic medical record. Surgical Oncology Clinics of North America, 24, 639–652. doi:10.1016/j.soc.2015.06.001
Wood, M.E., Kadlubek, P., Pham, T.H., Wollins, D.S., Lu, K.H., Weitzel, J.N., . . . Hughes, K.S. (2014). Quality of cancer family history and referral for genetic counseling and testing among oncology practices: A pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. Journal of Clinical Oncology, 32, 824–829.
Ziogas, A., & Anton-Culver, H. (2003). Validation of family history data in cancer family registries. American Journal of Preventive Medicine, 24, 190–198. doi:10.1016/S0749-3797(02)00593-7
About the Author(s)
Mahon is a professor in the Department of Internal Medicine in the Division of Hematology/Oncology and a professor of adult nursing in the School of Nursing at Saint Louis University in Missouri. No financial relationships to disclose. Mahon can be reached at mahonsm@slu.edu, with copy to editor at ONFEditor@ons.org.

