Cardiometabolic Health Among Cancer Survivors: A 13-Week Pilot Study of a Combined Aerobic and Resistance Training Program
Purpose/Objectives: To explore the feasibility of combined aerobic and resistance training (CART) as a safe method of improving cardiometabolic health among cancer survivors.
Design: Descriptive and longitudinal pilot study for exercise intervention.
Setting: University campus in Los Angeles, California.
Sample: A multiethnic population of cancer survivors (N = 11) was recruited by convenience sampling and physician referral.
Methods: Consenting participants were prescribed CART for one hour per day, three days per week for 13 weeks.
Main Research Variables: Components of cardiometabolic health were measured, including resting heart rate (HRrest), blood pressure, body mass index, waist circumference, body fat percentage, and android fat percentage at baseline and after 13 weeks of training. Fasting blood glucose, insulin, adiponectin, leptin, tumor necrosis factor alpha, and C-reactive protein (CRP) also were assessed at baseline and after 13 weeks of training.
Findings: More than half of the participants reported living with at least two other chronic diseases or conditions in addition to a cancer diagnosis. Five of six African American and Hispanic participants reported the presence of at least two risk factors for metabolic syndrome, compared to one of five Caucasian participants. After 13 weeks of training, participants experienced an average decrease in waist circumference. Decrease in waist circumference was associated with a decrease in CRP. A relationship also was suggested between number of exercise sessions attended and improvement in HRrest.
Conclusions: A CART intervention among cancer survivors should continue to be explored in a larger sample to establish efficacy and effectiveness at improving cardiometabolic health. Because of the higher risk of comorbidity among cancer survivors in comparison to cancer-free adults, improving cardiometabolic health is as important as monitoring cancer recurrence. A need exists for increased attention to the post-treatment cardiometabolic health of cancer survivors and also for examining potential cardiometabolic health disparities among non-Caucasian cancer survivors.
Implications for Nursing: CART may be a plausible alternative to reduce the risk of metabolic syndrome and improve cardiometabolic health among cancer survivors. Additional studies that continue to explore the efficacy and effectiveness of CART may provide more information to help nurses and physicians determine whether the cancer survivorship care plan should include an exercise-based alternative to intervene on cardiometabolic health.
Jump to a section
Early detection through cancer screening and the increased efficacy of cancer treatments have improved the chances of survival among patients with cancer (Siegel et al., 2012). As of 2014, the estimated prevalence of cancer survivors in the United States was 14.5 million, with about 64% being considered 5-year survivors and 15% being considered 20-year survivors (DeSantis et al., 2014). Cancer survivors are living longer but with a greater comorbid burden than similarly aged individuals without cancer. Previous research indicates that cancer survivors experience a higher burden of chronic disease, disability, and obesity compared to cancer-free adults (Leach et al., 2015; Tarleton, Ryan-Ibarra, & Induni, 2014). Additional studies specifically report that long-term survivors were more likely to have risk factors for type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) in comparison to their cancer-free counterparts (Daher, Daigle, Bhatia, & Durand, 2012; Underwood et al., 2012; Weaver et al., 2013).
T2DM and CVD are clinically defined by poor cardiometabolic health. Cardiometabolic health is influenced by level of adiposity and dependent on properly functioning cardiovascular and metabolic systems (see Figure 1). Adiposity in the central region of the body, often referred to as the android region, is particularly associated with components of metabolic syndrome, dyslipidemia, hypertension, and insulin resistance (Antuna-Puente, Feve, Fellahi, & Bastard, 2008; Galic, Oakhill, & Steinberg, 2010; Rasouli & Kern, 2008). Insulin resistance also is widely recognized as a late effect of chemotherapy and radiation therapy (Guinan et al., 2014; Rajendran, Abu, Fadl, & Byrne, 2013; Vergès, Walter, & Cariou, 2014). Treatment-related cardiotoxicity and radiation-related damage to the cardiovascular and respiratory systems also exacerbate the risk for CVD (Ewer & Ewer, 2010; Fuchs et al., 2003).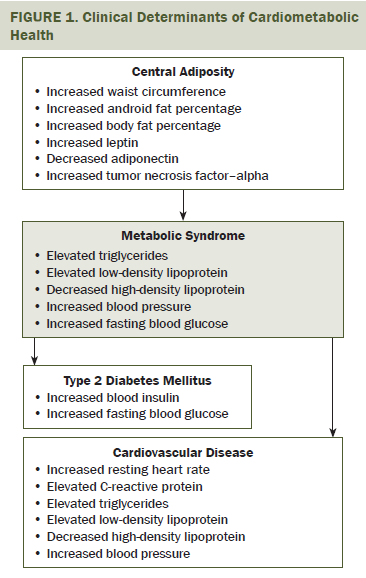
Because metabolic syndrome strongly predisposes patients to T2DM and CVD, it is emerging as one of the greatest concerns of oncology practitioners. The majority of published studies of metabolic syndrome among cancer survivors largely have focused on breast cancer survivors and the prevalence of obesity because of treatment-related effects on hormone status and ability to engage in physical activity. About 40% of breast cancer survivors are overweight or obese and, among this population, the prevalence of metabolic syndrome ranges from 50%–55% (Buttros Dde et al., 2013; Elme et al., 2013; Ortiz-Mendoza, de-la-Fuente-Vera, & Pérez-Chávez, 2014). Obesity and metabolic syndrome among survivors also can be accompanied by elevated levels of C-reactive protein (CRP), a pro-inflammatory risk factor for poor cardiovascular health (Buttros Dde et al., 2013).
One nonpharmacologic way to improve cardiometabolic health among cancer survivors is to prescribe aerobic exercise combined with resistance training. Moderate-intensity exercise supports health and improves quality of life among cancer survivors by reducing adiposity and biomarkers associated with T2DM and CVD (Anand et al., 2011; Lin et al., 2010; Lujan & DiCarlo, 2013). The combination of aerobic exercise and resistance training can promote healthy cardiovascular, endocrine, and musculoskeletal systems (Friedenreich, Neilson, & Lynch, 2010; Lee et al., 2012). Aerobic exercise and resistance training also may have positive effects on resting heart rate (HRrest) and blood pressure (BP) and may improve the cardiorespiratory health of post-treatment cancer survivors (Midtgaard et al., 2013; Schmitz et al., 2005). In addition, regular participation in an exercise program that includes resistance training may remediate late effects of treatment, such as fatigue and cardiotoxicity (Brown et al., 2011).
Previously published studies of training programs for cancer survivors vary in the exercise prescription and outcomes measured. Programs that prescribed moderate-intensity exercise consistently noted significant improvements in body composition, biomarkers, or cardiovascular health (Allgayer, Nicolaus, & Schreiber, 2004; Guinan et al., 2013; Hayes, Davies, Parker, & Bashford, 2003; Hsieh et al., 2008), whereas programs based on walking or low-intensity exercise did not report measurable change in these risk factors. Although the benefits of moderate-intensity exercise have been shown to be greater than those of low-intensity programs, the length of the prescribed programs and which outcomes are assessed vary. The majority of the previously published work focuses on weight loss or biomarkers (Allgayer et al., 2004) or on cardiovascular health (Broderick et al., 2013; Dimeo, Fetscher, Lange, Mertelsmann, & Keul, 1997; Hsieh et al., 2008) as independent outcomes. To the researchers’ knowledge, only one study has approached cardiometabolic health comprehensively and assessed the prevalence of metabolic syndrome among cancer survivors (Guinan et al., 2013).
The purpose of this pilot implementation of the improving physical activity after cancer treatment (IMPAACT) study is to evaluate whether 13 weeks of combined aerobic and resistance training (CART) could safely and feasibly improve cardiometabolic health and reduce comorbidities. This study also aims to assess the feasibility of analyzing all three of the cardiometabolic health domains (anthropometric, functional, and biomarker) and metabolic syndrome in the same study population as important outcomes for an exercise training intervention.
Methods
Design
The IMPAACT study was approved by the Loyola Marymount University (LMU) Institutional Review Board and conducted on LMU’s Westchester campus in Los Angeles, California. A multiethnic population of cancer survivors responded to recruitment by convenience sampling methods, which included survey distribution and flyers within a 15-mile radius of LMU’s campus, physician referral, and local community electronic mailing lists. Of the 24 survivors who expressed interest, 11 provided written consent to participate in the feasibility study. Reasons reported by 13 survivors for declining participation included transportation limitations, work schedules, lack of child care, and poor health. All cancer survivors who participated in the study provided written informed consent. Interested survivors were excluded if they were experiencing recurrent cancer or a new primary cancer, currently receiving radiation or chemotherapy, had a myocardial infarction or stroke within the past year, had lymphedema or inflammation of the pelvis or lower extremities, had a history of fainting while exercising, or had a history of chest pain or shortness of breath brought on by exercise. Participants were asked to inform their physicians of their planned participation and to review the exercise and assessment protocols with their physicians. Eleven participants were assessed at baseline by a registered clinical exercise physiologist to determine whether the exercise intervention was appropriate for their individual ability. Two participants withdrew from the study between baseline and postintervention assessments, citing increased family responsibilities. The remaining nine participants completed the 13-week intervention and the baseline and postintervention assessments.
Data Collection
Demographic characteristics, medical history, reproductive history, and physical activity patterns were obtained at baseline from a self-administered questionnaire. Cardiovascular health was assessed at baseline and postintervention by a registered clinical exercise physiologist as participants rested supine for 10 minutes in a quiet environment, free of sensory stimulation. HRrest and BP were recorded as the lowest HR during and as the average of two consecutive measurements at the conclusion of the rest period, respectively. Height, waist circumference, and weight were measured by exercise physiologists using a stadiometer (cm), tape measure (cm), and digital scale (kg), respectively, at baseline and postintervention. Waist circumference measurements are established as a practical, valid, and reliable method to quantify fat in the abdominal region and predict risk for chronic disease (Klein et al., 2007). Specifically, waist circumference was the perimeter measured at the narrowest circumference of the body between the lowest rib and the iliac crest. Participants wore minimal clothing, and measurements were taken after exhaling while arms hung freely. The tape measure, made of non-stretch material, was placed perpendicular to the long axis of the body with the tape snug, but not compressed, against skin. The Aerobic Center Longitudinal Study Physical Activity Questionnaire (Pereira et al., 1997) was used to assess regular physical activity for three months prior to study enrollment. This validated questionnaire is self-administered and allows for evaluation of intensity (in metabolic equivalents) and duration of regular physical activity (Kohl, Blair, Paffenbarger, Macera, & Kronenfeld, 1988).
Dual-energy x-ray absorptiometry (DXA) analysis of body composition was performed at baseline and postintervention. DXA of the whole body was used to quantify whole body fat percentage and fat percentage in the android body segment. The DXA analysis software defines the android region of interest as the approximate area around the waist between the mid-point of the lumbar spine and the superior border of the pelvis.
Fifteen milliliters of an overnight fasting blood sample were collected from each consenting participant by a licensed phlebotomist using venipuncture at baseline and postintervention time points. Fasting blood glucose (FBG) and insulin were assessed as risk factors for T2DM (Rynders et al., 2014). Adiponectin and leptin are adipokines produced by adipose tissue and were assessed as risk factors for T2DM and CVD (Diaz, Karlan, & Li, 2013). Tumor necrosis factor (TNF)–alpha and CRP are biomarkers of inflammation and were assessed as risk factors for CVD (Folsom, 2013; van Holten et al., 2013). Blood samples were processed at the LMU Biomedical Science laboratory by trained laboratory technicians for serum separation, aliquoted, and stored at –80°C. Levels of insulin (intra-assay 3% coefficient of variation [CV], inter-assay 11% CV), adiponectin (intra-assay 4% CV, inter-assay 10% CV), leptin (intra-assay 5% CV, inter-assay 13% CV), TNF-alpha (intra-assay 2.6% CV, inter-assay 13% CV), and CRP (intra-assay < 10% CV, inter-assay < 15% CV) were analyzed at the Norris Comprehensive Cancer Center at the University of Southern California in Los Angeles. FBG was assessed at the University of California, Los Angeles Clinical and Translational Research Laboratory on an Olympus AU400® chemistry immuno-analyzer platform (intra-assay < 2% CV, inter-assay < 3% CV).
Thresholds for biomarker assessments were set according to expected values identified in the literature. Participants could be classified at baseline as prediabetic based on an FBG of more than 100 mg/dl or a fasting blood insulin level of more than the recommended threshold of 20 mcU/ml, which is comparable to levels observed among individuals with T2DM and metabolic syndrome (García-Jiménez et al., 2015). Observed leptin levels of more than 20 ng/ml are within a range associated with being overweight or obese and insulin resistance (Zuo et al., 2013). Adiponectin levels of less than 15 mcg/ml are associated with being overweight or obese, and CRP levels of more than the threshold of 3 mg/L and less than 10 mg/L are considered to be at high risk for chronic inflammation (Ridker et al., 2008).
Cardiometabolic health was defined according to clinical criteria put forth by the National Heart, Lung, and Blood Institute at the National Institutes of Health (Nelms, Sucher, Lacey, & Roth, 2011). Based on these criteria, an individual with three or more of the following cardiometabolic risk factors can be clinically diagnosed with metabolic syndrome: a large waistline with increased risk, defined as greater than 88 cm for women and greater than 102 cm for men; triglyceride level of 150 mg/dl or greater; high-density lipoprotein cholesterol level of less than 50 mg/dl for women and less than 40 mg/dl for men; BP of 130/85 mmHg or greater; FBG of 100 mg/dl or greater; or currently taking medication for high cholesterol, high BP, or high FBG.
Exercise Intervention
The IMPAACT study is a supervised CART program prescribed for one hour per day, three days per week. Each exercise session includes: (a) 20 minutes of cardiorespiratory training; (b) 25 minutes of circuit-style combined resistance and cardiorespiratory training; and (c) 15 minutes of core training, flexibility exercises, and cool down. All exercise is prescribed in accordance with the American College of Sports Medicine (ACSM) exercise guidelines for cancer survivors (Schmitz et al., 2010). Prescribed cardiovascular intensity is determined using the Karvonen Formula (exercise target HR = [HR reserve] x percentage of exercise intensity + HRrest), in combination with medical history and current level of activity and physical fitness. HR reserve (maximal HR [HRmax] – HRrest) is a function of measured HRrest and HRmax, the latter of which is predicted based on participant’s age (HRmax = 220 – age).
During the CART program, cardiorespiratory training included walking or using an elliptical machine or stationary bike to achieve an individually prescribed target HR training zone (40%–80% of HR reserve) by referring to an HR monitor. Circuit training included sets of eight upper and lower body exercises. During week 1, two sets of circuit training were performed for two minutes for program acclimation and correction of form, and then decreased to one minute during week 2. Beginning with week 3, the circuit training was performed as three sets, with each set lasting 45 seconds, followed by a brief 20-second rest period. Core training involved two to three exercises to improve abdominal and trunk strength, which were accompanied by dynamic and static stretching to increase range of motion.
The exercises involved in the resistance circuit included different modes of upper- and lower-body resistance training, whole-body vibration, and lateral walking. Varied resistance or intensity was provided with elastic resistance bands, increasing step heights (4, 6, 8 inches), or varied body positions and body weight for resistance. All participants were provided customized options at each station to account for functional or injury limitations. Whole-body vibration was included to promote potential improvements in muscular strength (Lau et al., 2011), bone density (Slatkovska, Alibhai, Beyene, & Cheung, 2010), and neuropathy (Kessler & Hong, 2013). Training was performed on a Vibraflex® 550 platform (also called the Galileo® 2000). Vibration began at 30 seconds and 20 Hz for the first four weeks of the program and progressed to a 45-second duration at 25 Hz, with participants performing simple movements such as heel raises and partial squats concurrently during vibration exposure.
Statistical Analysis
Changes in anthropometrics, functional variables, and biomarkers were evaluated before and after 13 weeks of training. Simple relationships were assessed by Pearson’s correlation coefficients. All determinations were performed using SPSS®, version 18.2.
Results
Baseline Characteristics
The IMPAACT study volunteers were a multiethnic population, with more than half of the participants self-identifying as African American or Hispanic (see Table 1). Participants (N = 11) were largely female and breast cancer survivors without a history of metastatic disease, and the average cohort age was about 58 years. The majority of participants were less than five years since the time of their last treatment.
[[{"type":"media","view_mode":"media_original","fid":"23366","attributes":{"alt":"","class":"media-image","height":"596","typeof":"foaf:Image","width":"366"}}]]
The ACSM recommends at least 150 minutes of moderate-intensity physical activity each week (Schmitz et al., 2010). Based on responses collected using the Aerobics Center Longitudinal Physical Activity Questionnaire (Pereira et al., 1997), at baseline, 6 of 11 participants self-reported that their regular physical activity met ACSM guidelines. No participants were performing vigorous activity before enrollment. At baseline, two participants presented with at least three cardiometabolic risk factors and could have been clinically diagnosed with metabolic syndrome (see Table 2). More than half of participants reported gaining weight in the year prior to joining the study, with the most frequently reported gain being 5–10 pounds.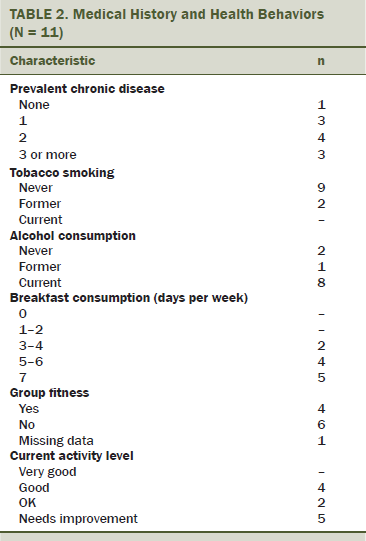
In addition to being diagnosed with cancer, 10 participants self-reported a clinical diagnosis of at least one other chronic disease or condition. Seven participants reported living with at least two other chronic diseases or conditions, in addition to being a cancer survivor. The most commonly reported chronic diseases and conditions at baseline included high BP (n = 2), thyroid conditions (n = 5), chronic bronchitis (n = 2), and osteoporosis or osteopenia (n = 2).
Cardiovascular Health and Body Composition
Cardiovascular variables, such as HRrest and BP, are displayed in Table 3. Four of 11 participants met the American Heart Association criteria for diagnosis of prehypertension (Midtgaard et al., 2013). Body composition and anthropometric data are presented for all participants and by race/ethnicity. At baseline, 5 of 11 participants were classified as overweight (body mass index [BMI] > 25 kg/m2) and another three were classified as obese (BMI > 30 kg/m2). When examining waist circumference at study entry, Caucasian women displayed a smaller waistline than African American women. 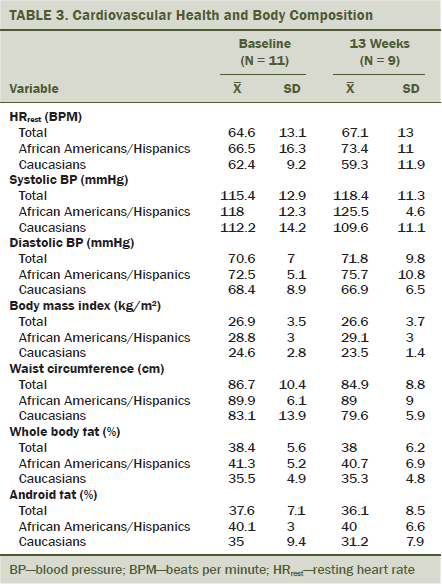
Metabolic Syndrome and Biomarkers of Cardiometabolic Health
Ten of 11 participants who completed the intervention provided fasting blood specimens for biomarker analysis. One participant declined to provide a blood specimen, citing a preexisting difficulty with giving blood because of venous damage from chemotherapy. At baseline, 3 of 10 participants who volunteered blood specimens could be classified as prediabetic based on an FBG of more than 100 mg/dl (mean = 112 mg/dl [SD = 12]), and one participant was at increased risk for prediabetes (FBG = 98 mg/dl). One participant had an elevated fasting blood insulin level of 22.9 mcU/ml, and one participant had a higher risk level of fasting blood insulin at 18.7 mcU/ml. Two participants had slightly increased leptin levels in the 15–20 ng/ml range, four participants had leptin levels of more than 20 ng/ml (mean = 35.4 ng/ml [SD = 17.1]), and five participants had adiponectin levels of less than 15 mcg/ml (mean = 11 mcg/dl [SD = 3.18]). One participant had a high-risk CRP level of 4.66 mg/L, and one participant had an elevated CRP level of 2.72 mg/L.
After 13 weeks of training, improvements were observed in prevalence of metabolic syndrome and in biomarkers of cardiometabolic health (see Tables 4 and 5). Decreases in waist circumference were associated with a decrease in CRP (r2 = 0.692). The largest decreases in CRP were observed among those with the greatest android fat mass at baseline (r2 = 0.826). Number of sessions attended was positively correlated with decreases in insulin (r2 = 0.72) and decreases in leptin (r2 = 0.785). Time since last treatment was positively correlated with decreases in insulin levels (r2 = 0.8) and FBG (r2 = 0.757). The researchers’ analysis also suggested that participants with the longest time since last treatment experienced the greatest decrease in interleukin-10 levels after training (r2 = 0.647). No relationships were observed between participation with adiponectin or TNF-alpha after 13 weeks of CART. 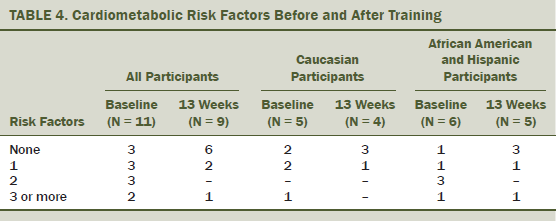
Postintervention Change in Cardiometabolic Health
Adherence to the prescribed exercise intervention (one hour per day, three days per week for 13 weeks, with 38 possible sessions) ranged from 24%–97%, with an average participation of 63% (SD = 27%). Reasons reported for missing sessions included changes in work schedules, childcare responsibilities, temporary illness (e.g., seasonal colds), and family vacations. Six of the 11 participants experienced a weight gain of 5–15 pounds in the year prior to study enrollment. Postintervention analysis showed an average decrease in waist circumference of 1.8 cm (SD = 3.97), with three of nine participants experiencing more than a 3 cm reduction in waist circumference. The participants with the largest decrease in waist circumference were also participants who attended more than 70% of the training sessions during the 13-week pilot study. As a whole, participants experienced an 11% decrease in average fat mass of the android body region. A trend of a decrease in android total mass, android fat percentage, and whole body fat percentage was seen. No significant change was observed from baseline to postintervention of HRrest or BP. However, a relationship was found between number of sessions attended and improvement in HRrest.
[[{"type":"media","view_mode":"media_original","fid":"23371","attributes":{"alt":"","class":"media-image","height":"471","typeof":"foaf:Image","width":"568"}}]]
No injuries were reported during the CART exercise sessions, and, during the 13-week IMPAACT program, no harm was reported by participants from the prescribed exercise.
Discussion
The IMPAACT study explored the preliminary efficacy of an exercise prescription for CART by assessing three domains of change in cardiometabolic health: anthropometric, functional, and biomarker. Through cross-sectional assessment in the small sample of multiethnic cancer survivors who had completed cancer treatment, the researchers found that 10 participants self-reported comorbid conditions of chronic disease. In addition, two participants were found to exhibit three or more risk factors of cardiometabolic health and could be diagnosed with metabolic syndrome.
The researchers explored the feasibility of whether a relatively short 13-week aerobic and resistance training intervention could influence cardiometabolic health and observed patterns of improvement in the anthropometric, functional, and biomarker-related risk factors for metabolic health within the study population. Improvements in waist circumference, body composition, and HRrest suggest that CART could be a safe and effective method of improving cardiometabolic health for cancer survivors. The relationships observed in this feasibility study between exercise participation and changes in biomarkers of cardiometabolic health suggest that repeating this intervention with a larger sample size and longer intervention would be worthwhile. With at least six months of training, visualizing temporal relationships between anthropometric and functional changes and improvements in cardiometabolic biomarkers may be possible (Ryan, Nicklas, Berman, & Elahi, 2003). Expanding this approach in a larger sample size also may allow for the analysis of demographic characteristics as mediators of potential temporal relationships.
Because of the racial homogeneity of previously published studies and the observed racial disparities in the baseline prevalence of metabolic syndrome in the current study population, a need exists for additional assessment of cardiometabolic health among non-Caucasian cancer survivors. Differences in risk and prevalence of T2DM, hypertension, and CVD have been observed in longitudinal and cross-sectional comparisons of people who are African American and Caucasian (Brancati, Kao, Folsom, Watson, & Szklo, 2000; Loehr et al., 2004). Disparities in comorbid burden have been reported among cancer survivors by race/ethnicity (Allard & Maxwell, 2009; Ashing, Rosales, Lai, & Hurria, 2014; Haynes & Smedley, 1999; Tammemagi, 2007; Winnick, Gaillard, & Schuster, 2008). Previous research established that T2DM was more prevalent among Hispanic cancer survivors, and CVD and circulatory complications were more prevalent among African American cancer survivors (Schultz, Stava, Beck, & Vassilopoulou-Sellin, 2004).
The IMPAACT study was able to assess cardiometabolic health in a multiethnic cohort of cancer survivors and identify potential racial disparities in prevalence of metabolic syndrome and in levels of biomarkers of cardiometabolic risk. In general, Caucasian female participants showed a trend for improvement in variables of cardiovascular health and body composition, which was not observed in African American female participants. However, because the researchers did not observe statistically significant disparities in this feasibility study, repeating the investigation in a larger multiethnic population will be important to quantify these potential relationships between race and cardiometabolic outcomes.
The IMPAACT study design represents a unique shift toward assessing the effect of training on cardiometabolic health, as represented by a profile that includes risk of metabolic syndrome, as well as abnormal levels of related biomarkers. In addition, the study also is innovative in using DXA-derived data in combination with anthropometric measures to objectively measure android fat mass, which is particularly relevant to T2DM and CVD. Although individual risk factors (e.g., BMI) are important to assess, the tendency to assess each risk factor independent of one another as risk factors for either T2DM or CVD may focus too narrowly on long-term outcomes. This may lead to a missed opportunity to intervene on cardiometabolic health in the early stages of metabolic syndrome, which would be of great benefit to this population of cancer survivors who already are experiencing greater morbidity in comparison to cancer-free adults.
Collectively, the researchers’ findings suggest that changes in markers of cardiometabolic health may be associated with improvements in body composition and cardiovascular health. These positive benefits appear to be most salient for those who recently have completed treatment. The findings of this feasibility study may be of particular interest to nursing professionals who work with patients at the conclusion of cancer treatment. Although walking is an important activity for maintaining fitness, additional emphasis is needed by healthcare providers on quantifying the benefits of combined programs of aerobic exercise and resistance training on metabolic risk factors and remediating late treatment effects. CART also may reduce the long-term risk for chronic disease after cancer treatment. Continuing to explore potential relationships between biomarkers, body composition, and cardiovascular health in response to exercise in larger, multiethnic populations of cancer survivors is important.
Limitations
The current study is limited by sample size, but it remains comparable to samples sizes reported in seven feasibility studies of cancer survivors (Balneaves et al., 2014; Campbell et al., 2012; Cormie et al., 2013; Frensham, Zarnowiecki, Parfitt, King, & Dollman, 2014; Katz et al., 2010; Peddle-McIntyre, Bell, Fenton, McCargar, & Courneya, 2012; Spector, Deal, Amos, Yang, & Battaglini, 2014). These studies also are comparable in the focus on piloting in-person or group-based exercise interventions with the goal of addressing treatment-related effects and comorbidity. The lack of comparison to a control group in the current study limits the ability to understand changes in cardiometabolic health after cancer treatment without exercise. However, because of the longitudinal design, each participant serves as his or her own control, with the baseline assessment of each participant providing a reference for cardiometabolic health. The short duration of the exercise intervention limits interpretation, but small positive improvements suggest that CART of longer duration may be beneficial to the cardiometabolic health of cancer survivors.
Conclusion and Implications for Nursing
Cancer survivorship is a distinct stage of the cancer experience and is highly dependent on sustainable health promotion after treatment. A comprehensive cancer survivorship plan can aid cancer survivors in enhancing the transition from treatment to the next life stage and improving treatment outcomes. Nurses have an opportunity to build relationships with cancer survivors because they see the same patients during different stages of treatment and follow-up. The role of oncology nurses in patient education in the areas of nutrition and lifestyle interventions has been established by the review of international research evidence, clinical literature, and analysis of current policy. A need exists for enhancement of professional guidance in nursing with regard to physical activity so that nurses know what to safely recommend to effectively promote good cardiometabolic health and to know when these recommendations may be most pertinent.
Because of prior findings and those of the current study, oncology nurses are well positioned to deliver guidance regarding physical activity and engagement in regular exercise as a means of mediating T2DM and CVD risk factors at the point of a patient’s transition from cancer treatment to post-treatment survivorship. 
The authors gratefully acknowledge the participants of the IMPAACT study; their research collaborators, Stephanie Perez, MS, ATC, Todd Shoepe, EdD, MS, CSCS, and Sarah Strand, PhD, ATC; their student research assistants, Daniel Conti, Claire Cronenweth, Isabela Kuroyama, Lacey Smith, and Samantha Weckmann; and Danielle Good-Dawson, BA, and Joshua Korte, BS, for their consistent administrative and laboratory support.
References
Allard, J.E., & Maxwell, G.L. (2009). Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control, 16, 53–56.
Allgayer, H., Nicolaus, S., & Schreiber, S. (2004). Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detection and Prevention, 28, 208–213.
Anand, S., Chertow, G.M., Johansen, K.L., Grimes, B., Kurella Tamura, M., Dalrymple, L.S., & Kaysen, G.A. (2011). Association of self-reported physical activity with laboratory markers of nutrition and inflammation: The comprehensive dialysis study. Journal of Renal Nutrition, 21, 429–437. doi:10.1053/j.jrn.2010.09.007
Antuna-Puente, B., Feve, B., Fellahi, S., & Bastard, J.P. (2008). Adipokines: The missing link between insulin resistance and obesity. Diabetes and Metabolism, 34, 2–11. doi:10.1016/j.diabet.2007.09.004
Ashing, K., Rosales, M., Lai, L., & Hurria, A. (2014). Occurrence of comorbidities among African-American and Latina breast cancer survivors. Journal of Cancer Survivorship, 8, 312–318. doi:10.1007/s11764-014-0342-x
Balneaves, L.G., Van Patten, C., Truant, T.L., Kelly, M.T., Neil, S.E., & Campbell, K.L. (2014). Breast cancer survivors’ perspectives on a weight loss and physical activity lifestyle intervention. Supportive Care in Cancer, 22, 2057–2065. doi:10.1007/s00520-014-2185-4
Brancati, F.L., Kao, W.H., Folsom, A.R., Watson, R.L., & Szklo, M. (2000). Incident type 2 diabetes mellitus in African American and white adults: The Atherosclerosis Risk in Communities Study. JAMA, 283, 2253–2259. doi:10.1001/jama.283.17.2253
Broderick, J.M., Guinan, E., Kennedy, M.J., Hollywood, D., Courneya, K.S., Culos-Reed, S.N., . . . Hussey, J. (2013). Feasibility and efficacy of a supervised exercise intervention in deconditioned cancer survivors during the early survivorship phase: The PEACH trial. Journal of Cancer Survivorship, 7, 551–562. doi:10.1007/s11764-013-0294-6
Brown, J.C., Huedo-Medina, T.B., Pescatello, L.S., Pescatello, S.M., Ferrer, R.A., & Johnson, B.T. (2011). Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiology, Biomarkers and Prevention, 20, 123–133. doi:10.1158/1055-9965.epi-10-0988
Buttros Dde, A., Nahas, E.A., Vespoli Hde, L., Uemura, G., de Almeida Bda, R., & Nahas-Neto, J. (2013). Risk of metabolic syndrome in postmenopausal breast cancer survivors. Menopause, 20, 448–454.
Campbell, K.L., Van Patten, C.L., Neil, S.E., Kirkham, A.A., Gotay, C.C., Gelmon, K.A., & McKenzie, D.C. (2012). Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. Journal of the Academy of Nutrition and Dietetics, 112, 559–567. doi:10.1016/j.jada.2011.10.022
Cormie, P., Newton, R.U., Spry, N., Joseph, D., Taaffe, D.R., & Galvão, D.A. (2013). Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer and Prostatic Diseases, 16, 328–335. doi:10.1038/pcan.2013.22
Daher, I.N., Daigle, T.R., Bhatia, N., & Durand, J.B. (2012). The prevention of cardiovascular disease in cancer survivors. Texas Heart Institute Journal, 39, 190–198.
DeSantis, C.E., Lin, C.C., Mariotto, A.B., Siegel, R.L., Stein, K.D., Kramer, J.L., . . . Jemal, A. (2014). Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians, 64, 252–271. doi:10.3322/caac.21235
Diaz, E.S., Karlan, B.Y., & Li, A.J. (2013). Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecologic Oncology, 129, 353–357. doi:10.1016/j.ygyno.2013.02.006
Dimeo, F., Fetscher, S., Lange, W., Mertelsmann, R., & Keul, J. (1997). Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood, 90, 3390–3394.
Elme, A., Utriainen, M., Kellokumpu-Lehtinen, P., Palva, T., Luoto, R., Nikander, R., . . . Saarto, T. (2013). Obesity and physical inactivity are related to impaired physical health of breast cancer survivors. Anticancer Research, 33, 1595–1602.
Ewer, M.S., & Ewer, S.M. (2010). Cardiotoxicity of anticancer treatments: What the cardiologist needs to know. Nature Reviews. Cardiology, 7, 564–575. doi:10.1038/nrcardio.2010.121
Folsom, A.R. (2013). Classical and novel biomarkers for cardiovascular risk prediction in the United States. Journal of Epidemiology, 23, 158–162. doi:10.2188/jea.JE20120157
Frensham, L.J., Zarnowiecki, D.M., Parfitt, G., King, S., & Dollman, J. (2014). The experiences of participants in an innovative online resource designed to increase regular walking among rural cancer survivors: A qualitative pilot feasibility study. Supportive Care in Cancer, 22, 1923–1929. doi:10.1007/s00520-014-2177-4
Friedenreich, C.M., Neilson, H.K., & Lynch, B.M. (2010). State of the epidemiological evidence on physical activity and cancer prevention. European Journal of Cancer, 46, 2593–2604. doi:10.1016/j.ejca.2010.07.028
Fuchs, I.B., Landt, S., Bueler, H., Kuehl, U., Coupland, S., Kleine-Tebbe, A., . . . Schaller, G. (2003). Analysis of HER2 and HER4 in human myocardium to clarify the cardiotoxicity of trastuzumab (Herceptin). Breast Cancer Research and Treatment, 82, 23–28. doi:10.1023/B:BREA.0000003916.39959.73
Galic, S., Oakhill, J.S., & Steinberg, G.R. (2010). Adipose tissue as an endocrine organ. Molecular and Cellular Endocrinology, 316, 129–139. doi:10.1016/j.mce.2009.08.018
García-Jiménez, S., Bernal Fernández, G., Martínez Salazar, M.F., Monroy Noyola, A., Toledano Jaimes, C., Meneses Acosta, A., . . . Sánchez-Alemán, M.A. (2015). Serum leptin is associated with metabolic syndrome in obese Mexican subjects. Journal of Clinical Laboratory Analysis, 29, 5–9. doi:10.1002/jcla.21718
Guinan, E., Hussey, J., Broderick, J.M., Lithander, F.E., O’Donnell, D., Kennedy, M.J., & Connolly, E.M. (2013). The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors—A pilot study. Supportive Care in Cancer, 21, 1983–1992. doi:10.1007/s00520-013-1743-5
Guinan, E.M., Connolly, E.M., Healy, L.A., Carroll, P.A., Kennedy, M.J., & Hussey, J. (2014). The development of the metabolic syndrome and insulin resistance after adjuvant treatment for breast cancer. Cancer Nursing, 37, 355–362.
Hayes, S., Davies, P.S., Parker, T., & Bashford, J. (2003). Total energy expenditure and body composition changes following peripheral blood stem cell transplantation and participation in an exercise programme. Bone Marrow Transplantation, 31, 331–338.
Haynes, M.A., & Smedley, B.D. (Eds.). (1999). The unequal burden of cancer: An assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, DC: National Academies Press.
Hsieh, C.C., Sprod, L.K., Hydock, D.S., Carter, S.D., Hayward, R., & Schneider, C.M. (2008). Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncology Nursing Forum, 35, 909–915. doi:10.1188/08.onf.909-915
Katz, E., Dugan, N.L., Cohn, J.C., Chu, C., Smith, R.G., & Schmitz, K.H. (2010). Weight lifting in patients with lower-extremity lymphedema secondary to cancer: A pilot and feasibility study. Archives of Physical Medicine and Rehabilitation, 91, 1070–1076. doi:10.1016/j.apmr.2010.03.021
Kessler, N.J., & Hong, J. (2013). Whole body vibration therapy for painful diabetic peripheral neuropathy: A pilot study. Journal of Bodywork and Movement Therapies, 17, 518–522. doi:10.1016/j.jbmt.2013.03.001
Klein, S., Allison, D.B., Heymsfield, S.B., Kelley, D.E., Leibel, R.L., Nonas, C., & Kahn, R. (2007). Waist circumference and cardiometabolic risk: A consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. American Journal of Clinical Nutrition, 85, 1197–1202. doi:10.1038/oby.2007.632
Kohl, H.W., Blair, S.N., Paffenbarger, R.S., Jr., Macera, C.A., & Kronenfeld, J.J. (1988). A mail survey of physical activity habits as related to measured physical fitness. American Journal of Epidemiology, 127, 1228–1239. doi:10.1097/00008483-198810000-00012
Lau, R.W., Liao, L.R., Yu, F., Teo, T., Chung, R.C., & Pang, M.Y. (2011). The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: A systematic review and meta-analysis. Clinical Rehabilitation, 25, 975–988. doi:10.1177/0269215511405078
Leach, C.R., Weaver, K.E., Aziz, N.M., Alfano, C.M., Bellizzi, K.M., Kent, E.E., . . . Rowland, J.H. (2015). The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. Journal of Cancer Survivorship, 9, 239–251. doi:10.1007/s11764-014-0403-1
Lee, I.M., Shiroma, E.J., Lobelo, F., Puska, P., Blair, S.N., & Katzmarzyk, P.T. (2012). Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet, 380(9838), 219–229.
Lin, C.Y., Chen, P.C., Kuo, H.K., Lin, L.Y., Lin, J.W., & Hwang, J.J. (2010). Effects of obesity, physical activity, and cardiorespiratory fitness on blood pressure, inflammation, and insulin resistance in the National Health and Nutrition Survey 1999–2002. Nutrition, Metabolism, and Cardiovascular Diseases, 20, 713–719. doi:10.1016/j.numecd.2009.06.005
Loehr, L.R., Espeland, M.A., Sutton-Tyrrell, K., Burke, G.L., Crouse, J.R., III, & Herrington, D.M. (2004). Racial differences in endothelial function in postmenopausal women. American Heart Journal, 148, 606–611. doi:10.1016/j.ahj.2004.04.032
Lujan, H.L., & DiCarlo, S.E. (2013). Physical activity, by enhancing parasympathetic tone and activating the cholinergic anti-inflammatory pathway, is a therapeutic strategy to restrain chronic inflammation and prevent many chronic diseases. Medical Hypotheses, 80, 548–552. doi:10.1016/j.mehy.2013.01.014
Midtgaard, J., Christensen, J.F., Tolver, A., Jones, L.W., Uth, J., Rasmussen, B., . . . Rørth, M. (2013). Efficacy of multimodal exercise-based rehabilitation on physical activity, cardiorespiratory fitness, and patient-reported outcomes in cancer survivors: A randomized, controlled trial. Annals of Oncology, 24, 2267–2273.
Nelms, M.N., Sucher, K.P., Lacey, K., & Roth, S.L. (2011). Nutrition therapy and pathophysiology (2nd ed.). Belmont, CA: Wadsworth Cengage Learning.
Ortiz-Mendoza, C.M., de-la-Fuente-Vera, T.A., & Pérez-Chávez, E. (2014). Metabolic syndrome in Mexican women survivors of breast cancer: A pilot study at a general hospital. Medical Archives, 68, 19–21. doi:10.5455/medarh.2014.68.19-21
Peddle-McIntyre, C.J., Bell, G., Fenton, D., McCargar, L., & Courneya, K.S. (2012). Feasibility and preliminary efficacy of progressive resistance exercise training in lung cancer survivors. Lung Cancer, 75, 126–132. doi:10.1016/j.lungcan.2011.05.026
Pereira, M.A., FitzerGerald, S.J., Gregg, E.W., Joswiak, M.L., Ryan, W.J., Suminski, R.R., . . . Zmuda, J.M. (1997). A collection of Physical Activity Questionnaires for health-related research. Medicine and Science in Sports and Exercise, 29(Suppl.), S1–S205.
Rajendran, R., Abu, E., Fadl, A., & Byrne, C.D. (2013). Late effects of childhood cancer treatment: Severe hypertriglyceridaemia, central obesity, non alcoholic fatty liver disease and diabetes as complications of childhood total body irradiation. Diabetic Medicine, 30, e239–e242. doi:10.1111/dme.12234
Rasouli, N., & Kern, P.A. (2008). Adipocytokines and the metabolic complications of obesity. Journal of Clinical Endocrinology and Metabolism, 93(Suppl. 1), S64–S73. doi:10.1210/jc.2008-1613
Ridker, P.M., Danielson, E., Fonseca, F.A., Genest, J., Gotto, A.M., Jr., Kastelein, J.J., . . . Glynn, R.J. (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine, 359, 2195–2207. doi:10.1056/NEJMoa0807646
Ryan, A.S., Nicklas, B.J., Berman, D.M., & Elahi, D. (2003). Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. International Journal of Obesity and Related Metabolic Disorders, 27, 1066–1071. doi:10.1038/sj.ijo.0802387
Rynders, C.A., Weltman, J.Y., Jiang, B., Breton, M., Patrie, J., Barrett, E.J., & Weltman, A. (2014). Effects of exercise intensity on postprandial improvement in glucose disposal and insulin sensitivity in prediabetic adults. Journal of Clinical Endocrinology and Metabolism, 99, 220–228. doi:10.1210/jc.2013-2687
Schmitz, K.H., Courneya, K.S., Matthews, C., Demark-Wahnefried, W., Galvao, D.A., Pinto, B.M., . . . Schwartz, A.L. (2010). American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise, 42, 1409–1426. doi:10.1249/MSS.0b013e3181e0c112
Schmitz, K.H., Holtzman, J., Courneya, K.S., Mâsse, L.C., Duval, S., & Kane, R. (2005). Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology, Biomarkers and Prevention, 14, 1588–1595.
Schultz, P.N., Stava, C., Beck, M.L., & Vassilopoulou-Sellin, R. (2004). Ethnic/racial influences on the physiologic health of cancer survivors. Cancer, 100, 156–164. doi:10.1002/cncr.11897
Siegel, R., DeSantis, C., Virgo, K., Stein, K., Mariotto, A., Smith, T., . . . Ward, E. (2012). Cancer treatment and survivorship statistics, 2012. CA: A Cancer Journal for Clinicians, 62, 220–241.
Slatkovska, L., Alibhai, S.M.H., Beyene, J., & Cheung, A.M. (2010). Effect of whole-body vibration on BMD: A systematic review and meta-analysis. Osteoporosis International, 21, 1969–1980.
Spector, D., Deal, A.M., Amos, K.D., Yang, H., & Battaglini, C.L. (2014). A pilot study of a home-based motivational exercise program for African American breast cancer survivors: Clinical and quality-of-life outcomes. Integrative Cancer Therapies, 13, 121–132. doi:10.1177/1534735413503546
Tammemagi, C.M. (2007). Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Current Opinion in Obstetrics and Gynecology, 19, 31–36.
Tarleton, H.P., Ryan-Ibarra, S., & Induni, M. (2014). Chronic disease burden among cancer survivors in the California Behavioral Risk Factor Surveillance System, 2009–2010. Journal of Cancer Survivorship, 8, 448–459. doi:10.1007/s11764-014-0350-x
Underwood, J.M., Townsend, J.S., Stewart, S.L., Buchannan, N., Ekwueme, D.U., Hawkins, N.A., . . . Fairley, T.L. (2012). Surveillance of demographic characteristics and health behaviors among adult cancer survivors—Behavioral risk factor surveillance system, United States, 2009. Morbidity and Mortality Weekly Report. Surveillance Summaries, 61, 1–23.
van Holten, T.C., Waanders, L.F., de Groot, P.G., Vissers, J., Hoefer, I.E., Pasterkamp, G., . . . Roest, M. (2013). Circulating biomarkers for predicting cardiovascular disease risk; A systematic review and comprehensive overview of meta-analyses. PLOS One, 8, e62080. doi:10.1371/journal.pone.0062080
Vergès, B., Walter, T., & Cariou, B. (2014). Endocrine side effects of anti-cancer drugs: Effects of anti-cancer targeted therapies on lipid and glucose metabolism. European Journal of Endocrinology, 170, R43–R55. doi:10.1530/eje-13-0586
Weaver, K.E., Foraker, R.E., Alfano, C.M., Rowland, J.H., Arora, N.K., Bellizzi, K.M., . . . Aziz, N.M. (2013). Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: A gap in survivorship care? Journal of Cancer Survivorship, 7, 253–261. doi:10.1007/s11764-013-0267-9
Winnick, J.J., Gaillard, T., & Schuster, D.P. (2008). Resistance training differentially affects weight loss and glucose metabolism of White and African American patients with type 2 diabetes mellitus. Ethnicity and Disease, 18, 152–156.
Zuo, H., Shi, Z., Yuan, B., Dai, Y., Wu, G., & Hussain, A. (2013). Association between serum leptin concentrations and insulin resistance: A population-based study from China. PLOS One, 8, e54615.
About the Author(s)
Grote is a visiting assistant professor, Almstedt is an associate professor, and Tarleton is an assistant professor, all in the Department of Health and Human Sciences at Loyola Marymount University in Los Angeles, CA. This research was funded by the Undergraduate Research Opportunity Program, Summer Undergraduate Research Program, Rains Research Fund, and University Honors Program at Loyola Marymount University. Multiplex assays were performed in the University of Southern California Immune Monitoring Core Facility that is supported, in part, by the National Cancer Institute Cancer Center Shared Grant award (P30CA014089). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Almstedt and Tarleton contributed to the conceptualization and design. Grote, Almstedt, and Tarleton completed the data collection and contributed to the analysis and the manuscript preparation. Tarleton can be reached at heather.tarleton@lmu.edu, with copy to editor at ONFEditor@ons.org. Submitted January 2015. Accepted for publication June 22, 2015.

