Accidental Fall Rates in Community-Dwelling Adults Compared to Cancer Survivors During and Post-Treatment: A Systematic Review With Meta-Analysis
Purpose/Objectives: To identify whether rates of accidental falls are greater for cancer survivors living in the community during or post-treatment than people with no history of cancer.
Data Sources: In a systematic literature review that was conducted in December 2013, MEDLINE®, EMBASE, PubMed, and Web of Science were searched for cancer or oncology and accidental falls in prospective and retrospective cohort and case-controlled studies. Studies were included if they were conducted in a community-dwelling adult population and excluded if they were conducted in acute hospitals and hospice.
Data Synthesis: Of 484 articles initially identified, 10 were included in the review. Of these, three included a control or comparator group and had comparable outcome measures to include in a meta-analysis. The risk ratio for falls for the group with cancer was 1.11.
Conclusions: Accidental fall rates in community-dwelling adults with a cancer diagnosis are greater than rates of falls in adults without cancer; this elevated rate remains after acute care is finished. Patients undergoing active treatment have greater rates of falls. Pain, fatigue, and deconditioning may affect fall rates in the longer term.
Implications for Nursing: Nurses have the capacity to reduce risk of falls in community-dwelling cancer survivors during or post-treatment through provision of information, advocacy, and support around pain and fatigue management and promotion of physical activity.
Jump to a section
People living with or after cancer have to deal with the physical, psychosocial, and economic consequences associated with the disease and its treatments (Spoelstra, Given, von Eye, & Given, 2010). Increased life expectancy also brings with it the potential for increased doses of treatment, magnifying side effects and adverse outcomes of such treatments. One potential sequela of cancer is an increased risk of accidental falls, compounding the age-related decline in physical capacity that negatively affects the ability to maintain balance. This may be a direct pathophysiologic consequence of the disease or a side effect of its treatment.
Some cancers directly involve bone, muscle, and nerves (either peripheral nerves or the central nervous system), all of which play an important part in the maintenance of balance and prevention of falls. Physical activity restrictions of survivors are well documented, initiating deconditioning processes that subsequently may increase fall rates (Deimling, Sterns, Bowman, & Kahana, 2007). Fatigue and pain are common in survivors and also may reduce the amount of physical activity that people with cancer undertake, compounding the deconditioning effect.
Physiologically, treatments for cancer can exacerbate fatigue, pain, loss of aerobic endurance, and limitations in neuromuscular function, resulting in a major impact on everyday life (Gilliam & St. Clair, 2011; Schmitz et al., 2010) and potentially increasing the risk of accidental falls. Cancer treatment also has been linked to muscle wasting (Demark-Wahnefried et al., 2001; Freedman et al., 2004; Harvie, Campbell, Baildam, & Howell, 2004), peripheral neuropathy in the feet (Kuroi & Shimozuma, 2004), and vestibular ototoxicity (Croes, Koop, van Gils, & Neef, 2012), all of which have an impact on balance and falls in older adults (Chen et al., 2009; Inokuchi, Matsusaka, Hayashi, & Shindo, 2007; Orr, 2010). Concurrent decreases in bone mineral density also may increase the risk of fractures from any fall event.
One-third of people aged 65 years and older in the general community will fall every year, with half of those falling multiple times (Tiedemann, Sherrington, Sturnieks, & Lord, 2012). For the many people living with cancer, the literature provides disparate results regarding the rate of accidental falls. For example, fall rates have been reported at 50% just prior to accessing palliative care services (Stone, Lawlor, Nolan, & Kenny, 2011); this may be because of the progression of the disease and higher dose of medications to treat cancer. However, once in palliative care, fall rates drop to 17% (O’Connell, Cockayne, Wellman, & Baker, 2005), perhaps because of lower mobility and confinement to a bed. Some evidence also links a cancer diagnosis to an increased fall rate in people in acute hospital situations (McCarter-Bayer, Bayer, & Hall, 2005). No consensus exists on accidental fall rates in community-dwelling survivors.
The aims of this systematic review were to identify whether rates of accidental falls were different for people living in the community during and post-treatment compared to those without a cancer diagnosis and to establish the foundations for additional experimental research in this area.
Methods
A systematic literature search of databases (MEDLINE®, EMBASE, PubMed, and Web of Science) was performed in December 2013 to identify articles written in English regarding accidental falls and cancer published previously, with no limits to dates. The search was performed using the Medical Subject Headings (MeSH) terms with cancer or oncology in combination with accidental falls. Additional articles were identified by manually checking the reference lists of included papers and doing a hand search of grey literature. Articles were stored in an EndNote library during the screening process, and duplicates were removed. Two researchers independently reviewed the articles for inclusion after reading titles, abstracts, or full text, as required. Authors were contacted to clarify whether inclusion criteria were met if this was not apparent after reading the full text. The number of articles included and excluded at each time point is recorded in Figure 1.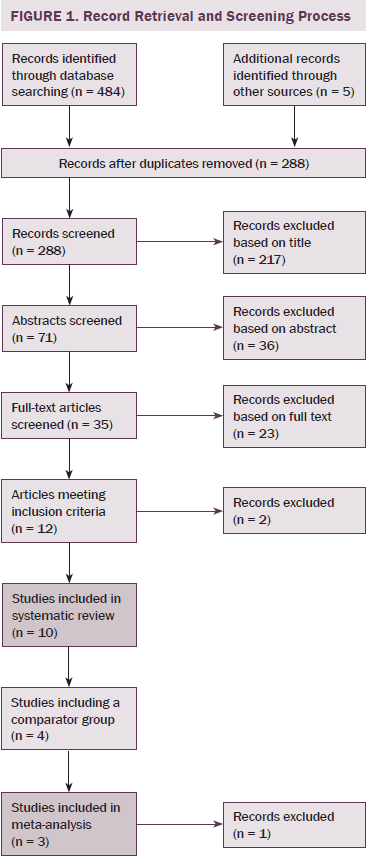
Articles were included if the population was adults living in the community who had been diagnosed with any type of cancer (excluding leukemia) and fall rates were included as an outcome measure. Data that were collected in acute hospital settings or palliative care were excluded. Study designs included prospective and retrospective observational and cohort studies, as well as case-controlled studies. This research project complied with the Declaration of Helsinki, with all of the articles reviewed having received ethical clearance from their relevant authorities.
Data Extraction
A standardized data extraction tool was developed by the research team to review the level of evidence and quality of the studies selected. In addition, information was collated on the number of participants, age at recruitment, description of groups that participated (e.g., cancer or no cancer), population characteristics (e.g., type of cancer, male or female), type of treatment received (e.g., radiation, chemotherapy), study setting, stage of cancer, and falls data outcomes for the groups. Any methodologic issues that may have given rise to bias also were recorded. Included studies were reviewed for bias under the following domains: selection, performance, detection, and reporting bias.
Two reviewers of the three available randomly were assigned to extract data from each article and did so independently. Subsequently, discussion among the reviewers took place to obtain an overall consensus on the data to be used for the systematic review.
Data Synthesis
Data from the studies that included a comparator group, which allowed comparison of fallers and those who did not report a fall, were synthesized from unadjusted data in a meta-analysis using RevMan, version 5.3. Despite a large variety in the ways in which the outcome of interest (fall rates) was described, risk ratio was calculated. Data were assessed for heterogeneity using I2 statistic.
Results
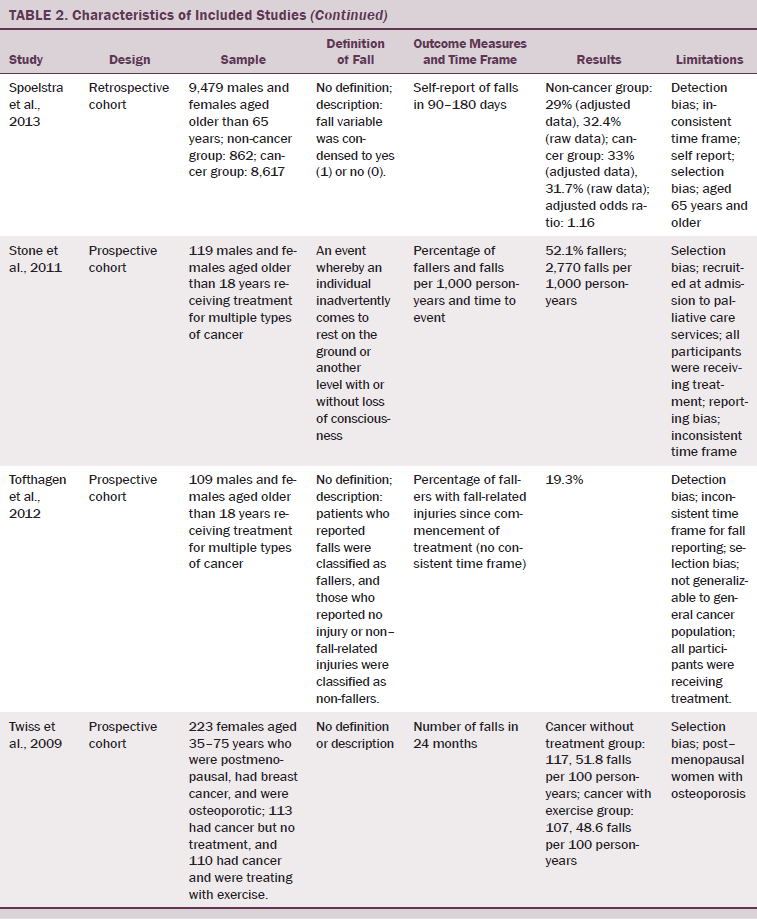
Of 484 publications identified in the initial search, 12 met inclusion criteria. Two were subsequently excluded; one reported on duplicate data, and one only reported data from participants who had suffered from an accidental fall. The remaining 10 were included in the authors’ systematic review. The study designs included retrospective cohort studies (Bylow et al., 2008; Spoelstra et al., 2013), several prospective cohort studies (Puts et al., 2013; Stone et al., 2011; Tofthagen, Overcash, & Kip, 2012; Twiss et al., 2009), observational prospective studies (Chen et al., 2009; Overcash & Beckstead, 2008), one observational retrospective study (Mohile et al., 2011), and one case-controlled observational study (Bylow et al., 2011).
Six of the 10 studies were based on a mixed gender population with multiple forms of cancer (Mohile et al., 2011; Overcash & Beckstead, 2008; Puts et al., 2013; Spoelstra et al., 2013; Stone et al., 2011; Tofthagen et al., 2012). The four remaining studies consisted of two studies of men with prostate cancer (Bylow et al., 2008, 2011) and two women-only studies, one comparing cancer with a control group (Chen et al., 2009) and one of which studied the effects of exercise on patients with breast cancer (Twiss et al., 2009). Sample sizes ranged from 50–146,959 patients, including seven smaller studies; five studies included fewer than 150 participants (Bylow et al., 2008, 2011; Puts et al., 2013; Stone et al., 2011; Tofthagen et al., 2012), one had 223 participants (Twiss et al., 2009), and another had 352 participants (Overcash & Beckstead, 2008). The majority of the population was aged 50 years and older, but two studies recruited participants with an age range of 18 years and older (Stone et al., 2011; Tofthagen et al., 2012), and a third study commenced its age range at 35 years (Twiss et al., 2009).
Four of the 10 studies included a control or comparison group (Chen et al., 2009; Mohile et al., 2011; Overcash & Beckstead, 2008; Spoelstra et al., 2010), with all other studies reporting on participants who had a cancer diagnosis only and comparing treatments (including chemotherapy and exercise) (Bylow et al., 2008, 2011; Puts et al., 2013; Stone et al., 2011; Tofthagen et al., 2012; Twiss et al., 2009). Three of these controlled studies recorded numbers of fallers and were included in the meta-analysis (see Table 1). The meta-analysis revealed an increased risk of falling in the survivor group (risk ratio: 1.11, 1.05–1.18, p = 0.0006). Heterogeneity was 90%.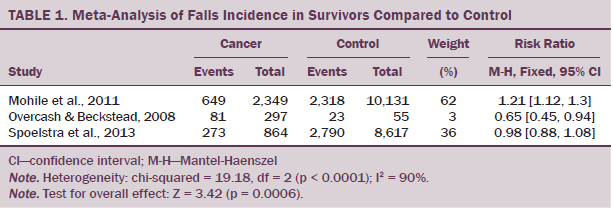
Falls
Comparison of fall data across studies was problematic because of lack of consistent definition and description of a fall, varying time frames for fall data collection, and the variation in the reporting of falls incidence data. Only 2 of the 10 articles included a definition of a fall (Overcash & Beckstead, 2008; Stone et al., 2011). Four articles provided no description of falls (Bylow et al., 2008, 2011; Puts et al., 2013; Twiss et al., 2009), one only recorded a fall if two or more falls were reported (Chen et al., 2009), and one only recorded falls that were injurious (Tofthagen et al., 2012). The remaining two articles described fallers if a fall had occurred and did not count actual falls (Mohile et al., 2011; Spoelstra et al., 2013). Time frames for data collection varied from three months to two years. In addition, the outcome measures for fall rates were described variously as percentage of fallers in 3 months (Bylow et al., 2008; Puts et al., 2013), 3–6 months (Spoelstra et al., 2013), 6 months (Bylow et al., 2011), or 12 months (Overcash & Beckstead, 2008; Mohile et al., 2011); annualized percentage of fallers if greater than two falls (Chen et al., 2009); percentage of fallers or falls per thousand person-years (Stone et al., 2011); percentage of fallers with fall-related injuries since commencement of treatment (no time frame) (Tofthagen et al., 2012); and number of falls in 24 months (Twiss et al., 2009).
Discussion
This meta-analysis indicates that accidental fall rates in community-dwelling adults with cancer are higher than in a comparable community-dwelling population. The review reports a high degree of variability in the rates of falls reported because of the heterogeneity of the populations with respect to type and stage of cancer, including current and past treatments. Multiple definitions of falls and ways of reporting (time frame and methods of data collection) are also factors that affect these results.
The meta-analysis of three studies (Mohile et al., 2011; Overcash & Beckstead, 2008; Spoelstra et al., 2013) (total number of participants = 169,996) provides evidence for a higher number of falls in survivors, but this must be interpreted with caution because a high level of heterogeneity and moderate bias exist in the included studies. The largest study in the current analysis (Mohile et al., 2011) was highly weighted in the model because of its large sample size of 12,480, but the differences in fall rates between the groups were quite small (26% for the cancer group and 22% for the non-cancer group). Of note, these rates are lower than would be expected in a similar-aged population (65 years and older), which may reflect that data used in the current study were collected from a large database. The smallest study (N = 352) (Overcash & Beckstead, 2008) included in the current meta-analysis found a lower rate of falling in the cancer group, but the authors used as a comparator patients from a general practice clinic with three or more comorbidities (mean = 5.9 comorbidities), potentially introducing selection bias into that study. The comorbidity level of those with cancer was not described in that study. Spoelstra et al. (2013) reported higher numbers of fallers in the cancer group (odds ratio = 1.16; 33% compared to 29% in three to six months), using data that had been adjusted for cognitive impairment, weight loss, pain, and number of comorbidities. The health status of the comparator groups and comorbidities of the cancer group in this study appear to be influential in these results.
The variation in falls rates in patients with cancer described in the current meta-analysis and review may be attributed to the time frames used to record fall data and overall health and functional status of the groups. The study that reported higher rates of falls recorded data during a relatively short time frame of three to six months. The annualized figure for this group may be inferred to be much higher, but the large variation in data collection time frames precludes meaningful extrapolation. Differences in how falls were described to and by participants also may be a confounding factor, but because two articles in the meta-analysis did not provide a definition of a fall, the magnitude of this is unknown. The inclusion of standardized reporting measures in relation to falls in additional studies, similar to the Prevention of Falls Network Europe recommendations (Lamb, Jrstad-Stein, Hauer, & Becker, 2005), would allow better comparison and interpretation of studies, improving the strength of evidence in this area and reducing the variability in reported fall rates within a population.
Other reasons for the differences in fall rates among the 10 studies included in the current review may relate to attributes of the populations studied in terms of cancer type and treatment, and the resultant functional abilities of those people. Although some studies included participants with any cancer, three studies included only people with prostate cancer (Bylow et al., 2008, 2011) or breast cancer (Twiss et al., 2009). Two studies recruited people aged older than 18 years, but most of the eligible studies included only older adults (aged older than 50 years or post-menopausal). No articles reported details on bone or neural tumors alone; therefore, no conclusions can be made regarding the role of these cancers as causal factors for falls.
Side effects of chemotherapy treatments can be peripheral neuropathy (Kuroi & Shimozuma, 2004), which affects the quality of sensory information required for balance control and muscle output in the lower limbs to maintain stability. One article included in the review but not the meta-analysis recruited participants who were undergoing treatment and had at least one sign of peripheral neuropathy (Tofthagen et al., 2012). The authors reported a rate of 19% for injurious falls; however, the rate of total falls in this study was not reported. This appears to be comparable with previously published data for injurious fall rates in older adults of 13% for men and 27% for women (Luukinen, Koski, Honkanen, & Kivelä, 1995). Two studies in the current review included details of the treatments undertaken and reported higher rates of falling in survivors undergoing chemotherapy compared to survivors not undertaking treatment (Bylow et al., 2011; Overcash & Beckstead, 2008).
Deconditioning, caused by treatment-induced fatigue, is one risk factor for falls of which oncology nurses should be aware (Holley, 2002). Cancer and its treatment are linked to fatigue, with its prevalence being reported as much as 90% (Prue, Rankin, Allen, Gracey, & Cramp, 2006), with ongoing fatigue reported (de Jong, Courtens, Abu-Saad, & Schouten, 2002). This may persist for years in as many as 75% of survivors (Daniëls, Oerlemans, Krol, van de Poll-Franse, & Creutzberg, 2013) and may affect physical performance and result in deconditioning because of lack of ability to engage in usual activities. Physical deconditioning in the general older population has been attributed to an increase in fall rate and injuries (Gregg, Pereira, & Caspersen, 2000), with increased rates of falling significantly associated with limitations in physical activity caused by a health problem (O’Loughlin, Robitaille, Boivin, & Suissa, 1993). Large limitations of physical activity after cancer diagnosis have been reported, with reductions in people meeting recommended exercise guidelines from 70% before breast cancer diagnosis to 39% after treatment, and further reducing to 15% after a second round of treatment (Andrykowski, Beacham, & Jacobsen, 2007). Health practitioners have a role in providing information and encouraging appropriate physical activity to prevent deconditioning and subsequent adverse outcomes. The American Cancer Society provides detailed exercise guidelines that include advice regarding maintenance of physical activity for people with cancer, even in the acute stages of treatment, to prevent deconditioning (Kushi et al., 2006). They do not include recommendations for balance training for falls prevention.
Cancer pain has been shown to be a strongly significant factor in accidental falls (Stone, Lawlor, Savva, Bennett, & Kenny, 2012). Pain of sufficient magnitude to interfere with daily activities also has been identified as a factor that increases fall rates in the general community by an adjusted prevalence ratio of 1.42 (p = 0.0001) (Blyth, Cumming, Mitchell, & Wang, 2007). Pain associated with cancer also may restrict physical activity, aggravating the fall risk effect. Healthcare professionals have an important role to play in assisting people with cancer to manage their pain effectively to minimize the effect on activities of daily living.
The manner in which fall data were recorded introduces the possibility of under- and over-reporting. Studies relied on self-reporting (Bylow et al., 2008; Overcash & Beckstead, 2008; Tofthagen et al., 2012; Twiss et al., 2009), completion of questionnaires with recall ranging from three to six months to two years (Bylow et al., 2011; Chen et al., 2009; Puts et al., 2013), or samples taken from large medical databases (Mohile et al., 2011; Spoelstra et al., 2013). Using large databases containing healthcare records for evaluating falls in the community introduces a potential reporting bias. Previous evaluation of this method has shown that less than 20% of falls are recorded by patients (Haga et al., 1996). In contrast, in one study included in the current review, weekly phone calls or in-person interviews were conducted with participants during a six-month period to help eliminate problems with recall over a longer period of time (Stone et al., 2012). In Stone et al. (2012), people with advanced cancer reported a higher fall rate of at least 50% during a six-month period.
All studies included in the current review presented with some degree of bias (see Table 2). Six studies (Bylow et al., 2008, 2011; Chen et al., 2009; Overcash & Beckstead, 2008; Spoelstra et al., 2013; Tofthagen et al., 2012) were deemed to have detection bias. Lack of definitions of outcome measures and the lack of the comparability of these measures (i.e., measuring two falls or injurious falls will not provide the same results as measuring one fall) were included as reasons for allocating this bias to those studies. Selection bias was allocated to seven studies (Chen et al., 2009; Mohile et al., 2011; Overcash & Beckstead, 2008; Puts et al., 2013; Stone et al., 2011; Tofthagen et al., 2012; Twiss et al., 2009). Limitations to age and gender in recruitment and having populations including only participants undergoing active treatment resulted in a lack of generalizability of results from these selected populations. Inconsistent intra-study time frames for these outcome measures produced reporting bias in two studies (Stone et al., 2011; Tofthagen et al., 2012). Heterogeneity was high because of different populations studied, types of cancer, stages of the disease process, functional levels, and definitions of events.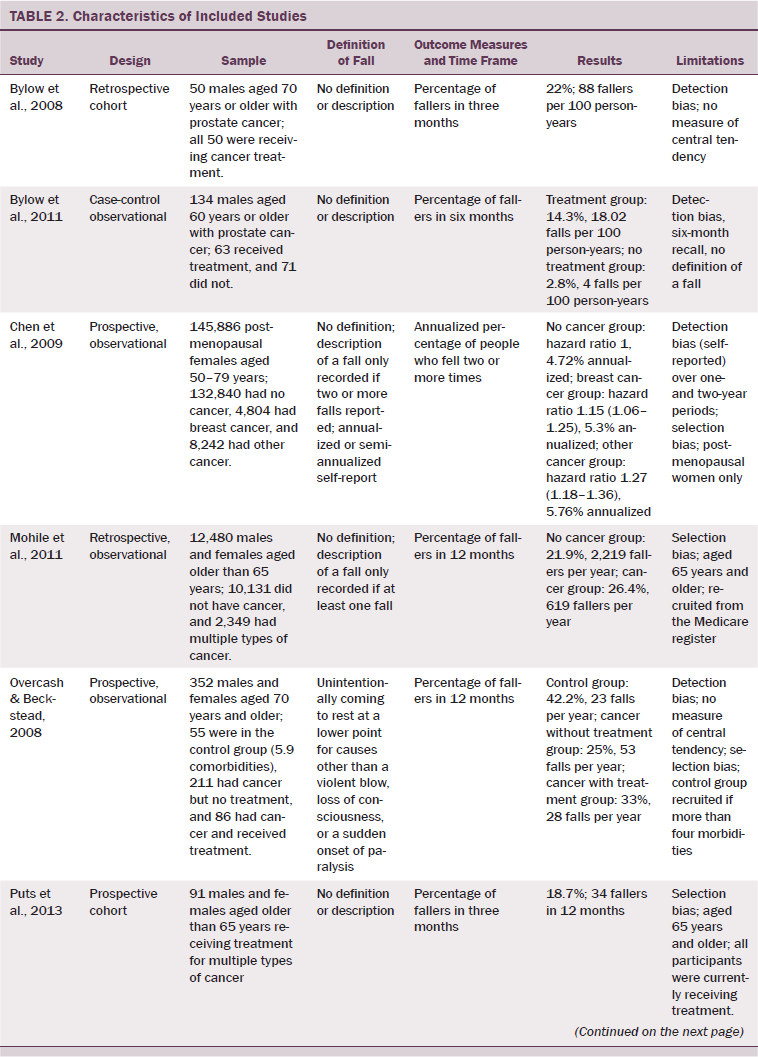
To allow for more effective analysis of additional studies, consistency across the method of recording fall outcomes, terminology that allows comparison across time frames of different studies (e.g., falls per 1,000 person-years), and use of a standard definition of an accidental fall will allow a fuller comparison of fall rates between survivors living in the community and the healthy population.
Conclusion
The current meta-analysis indicates that accidental fall rates in community-dwelling adults with cancer are higher than in a comparable community-dwelling population. The current review indicates that reasons for increased rate of falls in survivors may include factors pertaining to the functional level of the people during or post-treatment, pain, fatigue, and any treatment that they are undertaking.
Implications for Nursing
Fall rates in people with cancer are high, and oncology and community nurses can play a role in identifying and reducing these adverse events. Nurses are well placed to identify people with cancer who may be at higher risk for falls as a result of their physical functioning or treatment stage, and to provide education, advocacy, and support. Nurses need to be aware of falls risk factors that particularly affect patients with cancer, including fatigue, pain, and deconditioning. The level of fatigue in patients with cancer is high and may lead to deconditioning through inactivity. Nurses can provide advice and encouragement about maintaining daily activities and helping patients address barriers to physical activity by providing practical problem-solving suggestions. The therapeutic relationship that nurses develop with their patients with cancer facilitates communication about levels of pain, leading to more effective pain management and assisting in the prevention of accidental falls. Nurses need to closely monitor patients undergoing active cancer treatment (e.g., chemotherapy) because this may require specific advice to clients and modification of activities or surroundings. The continuity of care provided by nurses means that they are a valuable source of information and are in a unique position to maintain and improve levels of physical activity in people with cancer, thereby reducing their risk of falls (Loprinzi, Cardinal, Winters-Stone, Smit, & Loprinzi, 2012).
The authors gratefully acknowledge Shandell Elmer, PhD, RN, for her contribution to the nursing implication section of the article.
References
Andrykowski, M.A., Beacham, A.O., & Jacobsen, P.B. (2007). Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiology, Biomarkers, and Prevention, 16, 430–438. doi:10.1158/1055-9965.EPI-06-0735
Blyth, F.M., Cumming, R., Mitchell, P., & Wang, J.J. (2007). Pain and falls in older people. European Journal of Pain, 11, 564–571. doi:10.1016/j.ejpain.2006.08.001
Bylow, K., Dale, W., Mustian, K., Stadler, W.M., Rodin, M., Hall, W., . . . Mohile, S.G. (2008). Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology, 72, 422–427. doi:10.1016/j.urology.2008.03.032
Bylow, K., Hemmerich, J., Mohile, S.G., Stadler, W.M., Sajid, S., & Dale, W. (2011). Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: A case-control study. Urology, 77, 934–940. doi:10.1016/j.urology.2010.11.024
Chen, Z., Maricic, M., Aragaki, A.K., Mouton, C., Arendell, L., Lopez, A.M., . . . Chlebowski, R.T. (2009). Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: Results from the Women’s Health Initiative. Osteoporosis International, 20, 527–536. doi:10.1007/s00198-008-0721-0
Croes, S., Koop, A.H., van Gils, S.A., & Neef, C. (2012). Efficacy, nephrotoxicity and ototoxicity of aminoglycosides, mathematically modelled for modelling-supported therapeutic drug monitoring. European Journal of Pharmaceutical Sciences, 45, 90–100. doi:10.1016/j.ejps.2011.10.022
Daniëls, L.A., Oerlemans, S., Krol, A.D.G., van de Poll-Franse, L.V., & Creutzberg, C.L. (2013). Persisting fatigue in Hodgkin lymphoma survivors: A systematic review. Annals of Hematology, 92, 1023–1032. doi:10.1007/s00277-013-1793-2
Deimling, G.T., Sterns, S., Bowman, K.F., & Kahana, B. (2007). Functioning and activity participation restrictions among older adult, long-term cancer survivors. Cancer Investigation, 25, 106–116. doi:10.1080/07357900701224813
de Jong, N., Courtens, A.M., Abu-Saad, H.H., & Schouten, H.C. (2002). Fatigue in patients with breast cancer receiving adjuvant chemotherapy: A review of the literature. Cancer Nursing, 25, 283–297. doi:10.1097/00002820-200208000-00004
Demark-Wahnefried, W., Peterson, B.L., Winer, E.P., Marks, L., Aziz, N., Marcom, P.K., . . . Rimer, B.K. (2001). Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. Journal of Clinical Oncology, 19, 2381–2389.
Freedman, R.J., Aziz, N., Albanes, D., Hartman, T., Danforth, D., Hill, S., . . . Yanovski, J.A. (2004). Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. Journal of Clinical Endocrinology and Metabolism, 89, 2248–2253. doi:10.1210/jc.2003-031874
Gilliam, L.A., & St. Clair, D.K. (2011). Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxidants and Redox Signaling, 15, 2543–2563. doi:10.1089/ars.2011.3965
Gregg, E.W., Pereira, M.A., & Caspersen, C.J. (2000). Physical activity, falls, and fractures among older adults: A review of the epidemiologic evidence. Journal of the American Geriatrics Society, 48, 883–893. doi:10.1111/j.1532-5415.2000.tb06884.x
Haga, H., Yasumura, S., Niino, N., Ueno, H., Oshima, M., & Higuchi, Y. (1996). An examination of two reporting methods of falls among the elderly living in the community. Nihon Koshu Eisei Zasshi, 43, 983–988.
Harvie, M.N., Campbell, I.T., Baildam, A., & Howell, A. (2004). Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Research and Treatment, 83, 201–210. doi:10.1023/B:BREA.0000014037.48744.fa
Holley, S. (2002). A look at the problem of falls among people with cancer. Clinical Journal of Oncology Nursing, 6, 193–197.
Inokuchi, S., Matsusaka, N., Hayashi, T., & Shindo, H. (2007). Feasibility and effectiveness of a nurse-led community exercise programme for prevention of falls among frail elderly people: A multi-centre controlled trial. Journal of Rehabilitation and Medicine, 39, 479–485. doi:10.2340/16501977-0080
Kuroi, K., & Shimozuma, K. (2004). Neurotoxicity of taxanes: Symptoms and quality of life assessment. Breast Cancer, 11, 92–99.
Kushi, L.H., Byers, T., Doyle, C., Bandera, E.V., McCullough, M., Gansler, T., . . . Thun, M.J. (2006). American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA: A Cancer Journal for Clinicians, 56, 254–281. doi:10.3322/canjclin.56.5.254
Lamb, S.E., Jrstad-Stein, E.C., Hauer, K., & Becker, C. (2005). Development of a common outcome data set for fall injury prevention trials: The Prevention of Falls Network Europe consensus. Journal of the American Geriatrics Society, 53, 1618–1622. doi:10.1111/j.1532-5415.2005.53455.x
Loprinzi, P.D., Cardinal, B.J., Winters-Stone, K., Smit, E., & Loprinzi, C.L. (2012). Physical activity and the risk of breast cancer recurrence: A literature review. Oncology Nursing Forum, 39, 269–274. doi:10.1188/12.ONF.269-274
Luukinen, H., Koski, K., Honkanen, R., & Kivelä, S.L. (1995). Incidence of injury-causing falls among older adults by place of residence: A population-based study. Journal of the American Geriatrics Society, 43, 871–876. doi:10.1111/j.1532-5415.1995.tb05529.x
McCarter-Bayer, A., Bayer, F., & Hall, K. (2005). Preventing falls in acute care: An innovative approach. Journal of Gerontological Nursing, 31, 25–33. doi:10.3928/0098-9134-20050301-07
Mohile, S.G., Fan, L., Reeve, E., Jean-Pierre, P., Mustian, K., Peppone, L., . . . Dale, W. (2011). Association of cancer with geriatric syndromes in older Medicare beneficiaries. Journal of Clinical Oncology, 29, 1458–1464. doi:10.1200/JCO.2010.31.6695
O’Connell, B., Cockayne, M., Wellman, D., & Baker, L. (2005). Fall risk factors and the nature of falls in inpatient oncology and palliative care settings. Contemporary Nurse, 18, 247–257.
O’Loughlin, J.L., Robitaille, Y., Boivin, J.F., & Suissa, S. (1993). Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. American Journal of Epidemiology, 137, 342–354.
Orr, R. (2010). Contribution of muscle weakness to postural instability in the elderly. A systematic review. European Journal of Physical Rehabilitation and Medicine, 46, 183–220.
Overcash, J.A., & Beckstead, J. (2008). Predicting falls in older patients using components of a comprehensive geriatric assessment. Clinical Journal of Oncology Nursing, 12, 941–949. doi:10.1188/08.CJON.941-949
Prue, G., Rankin, J., Allen, J., Gracey, J., & Cramp, F. (2006). Cancer-related fatigue: A critical appraisal. European Journal of Cancer, 42, 846–863. doi:10.1016/j.ejca.2005.11.026
Puts, M.T., Monette, J., Girre, V., Wolfson, C., Monette, M., Batist, G., & Bergman, H. (2013). The fall rate of older community-dwelling cancer patients. Supportive Care in Cancer, 21, 775–783. doi:10.1007/s00520-012-1579-4
Schmitz, K.H., Courneya, K.S., Matthews, C., Demark-Wahnefried, W., Galvao, D.A., Pinto, B.M., . . . Schwartz, A.L. (2010). American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine Science and Sports Exercise, 42, 1409–1426. doi:10.1249/MSS.0b013e3181e0c112
Spoelstra, S., Given, B., von Eye, A., & Given, C. (2010). Falls in the community-dwelling elderly with a history of cancer. Cancer Nursing, 33, 149–155. doi:10.1097/NCC.0b013e3181bbbe8a
Spoelstra, S.L., Given, B.A., Schutte, D.L., Sikorskii, A., You, M., & Given, C.W. (2013). Do older adults with cancer fall more often? A comparative analysis of falls in those with and without cancer. Oncology Nursing Forum, 40, E69–E78. doi:10.1188/13.ONF.E69-E78
Stone, C., Lawlor, P.G., Nolan, B., & Kenny, R.A. (2011). A prospective study of the incidence of falls in patients with advanced cancer. Journal of Pain and Symptom Management, 42, 535–540.
Stone, C.A., Lawlor, P.G., Savva, G.M., Bennett, K., & Kenny, R.A. (2012). Prospective study of falls and risk factors for falls in adults with advanced cancer. Journal of Clinical Oncology, 30, 2128–2133. doi:10.1200/JCO.2011.40.7791
Tiedemann, A., Sherrington, C., Sturnieks, D., & Lord, S. (2012). Fall prevention. In F. Mooren (Ed.), Encyclopedia of exercise medicine in health and disease (pp. 331–334). Springer Berlin-Heidelberg.
Tofthagen, C., Overcash, J., & Kip, K. (2012). Falls in persons with chemotherapy-induced peripheral neuropathy. Supportive Care in Cancer, 20, 583–589. doi:10.1007/s00520-011-1127-7
Twiss, J.J., Waltman, N.L., Berg, K., Ott, C.D., Gross, G.J., & Lindsey, A.M. (2009). An exercise intervention for breast cancer survivors with bone loss. Journal of Nursing Scholarship, 41, 20–27.
About the Author(s)
Bird is a lecturer, Cheney is an exercise physiologist, and Williams is a senior lecturer, all in the School of Health Sciences at the University of Tasmania in Launceston, Australia. No financial relationships to disclose. Bird, Cheney, and Williams all contributed to the conceptualization and design, data collection, statistical support, analysis, and manuscript preparation. Bird can be reached at birdm@utas.edu.au, with copy to editor at ONFEditor@ons.org. Submitted May 2015. Accepted for publication July 30, 2015.

