Genetic Variants Influencing Patient Response to Opioid Therapy
Almost every nurse can relate to examples in which an individual receiving a high dose of an opioid had no analgesic effect, whereas another experienced respiratory compromise with a small dose. In many cases, variant responses can be attributed to genetic influences responsible for metabolism of the opioid through the cytochrome p450 system (CYP), including CYP2D6, CYP3A, and CYP2B6. Variants of the mu receptor can also affect the affinity of the opioid for its receptor. A discussion of some common alleles (polymorphisms) associated with variable response to opioids is included in this article.
Jump to a section
Almost every nurse can relate to examples in which an individual receiving a high dose of an opioid had no analgesic effect, whereas another experienced respiratory compromise with a small dose. In many cases, variant responses can be attributed to genetic influences responsible for metabolism of the opioid through the cytochrome p450 system (CYP), including CYP2D6, CYP3A, and CYP2B6. Variants of the mu receptor can also affect the affinity of the opioid for its receptor. A discussion of some common alleles (polymorphisms) associated with variable response to opioids is included in this article.
Phase I Metabolizing Enzymes
The CYP system is a group of more than 50 metabolizing enzymes responsible for the oxidation of endogenous and exogenous organic compounds (Cavallari, Jeong, & Bress, 2011; Eggert & Howe, 2010; Krau, 2013). Each enzyme within a given subfamily is encoded by a single gene, and polymorphisms are associated with each of these CYP enzymes (Krau, 2013). A polymorphism is a change in the gene’s DNA sequence that affects the gene’s protein product; each polymorphism associated with that gene is known as an allele. A person who receives the same allele from each parent is considered to be a homozygote at this gene location (locus). A person who receives a different allele from each parent is considered to be a heterozygote.
For each of the identified CYP enzymes, individuals can be placed into four functional categories known as phenotypes: poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers (EMs), and ultra metabolizers (UMs) (Cavallari et al., 2011; Fernandez Robles, Degnan, & Candiotti, 2012; Krau, 2013; Vuilleumier, Stamer, & Landau, 2012). PMs have two nonfunctional alleles and produce virtually no functional amount of that particular CYP enzyme. IMs have reduced enzyme activity, with at least one allele producing some functional enzyme. EMs carry two functional alleles, and they are considered to have normal enzyme activity. UMs have more than two active alleles (they have inherited extra copies of the gene in question), and these individuals express high enzyme activity. Because the production of each CYP enzyme is regulated by a single gene, an individual may have the IM phenotype for CYP3A4 and the UM phenotype for CYP2D6.
CYP2D6: CYP2D6 is responsible for metabolizing 20%–30% of all marketed drugs, including opioids (Cavallari et al., 2011; Fernandez Robles et al., 2012; Krau, 2013). CYP2D6 is the most polymorphic of the CYP system, with more than 130 alleles identified to date (Human Cytochrome P450 [CYP] Allele Nomenclature Committee, 2014). Among the most studied alleles are CYP2D6*1 and CYP2D6*2, which are associated with normal enzyme production, and CYP2D6*10, CYP2D6*17, CYP2D6*29, and CYP2D6*41, which are associated with decreased enzyme activity (Cavallari et al., 2011). CYP2D6*3, CYP2D6*4, CYP2D6*5, CYP2D6*6, CYP2D6*7, and CYP2D6*8 are identified as nonfunctional alleles (Cavallari et al., 2011; Ma & Lu, 2011). The distribution of these alleles varies among ethnic populations, and the individual’s phenotype will be determined by which two alleles have been inherited from his or her parents and if he or she inherited an extra copy of that same allele. The phenotypes with greatest implications to drug therapy are PMs and UMs. About 8%–10% of Caucasians and 50% of Asians are considered to be CYP2D6 PMs (Miaskowski, 2009), whereas 10%–16% of Saudi Arabians and Ethiopians are considered to be UMs (Cavallari et al., 2011; Krau, 2013). The response to therapy depends on whether the opioid is an active compound or a prodrug. Table 1 lists various opioid responses based on the patient’s phenotype.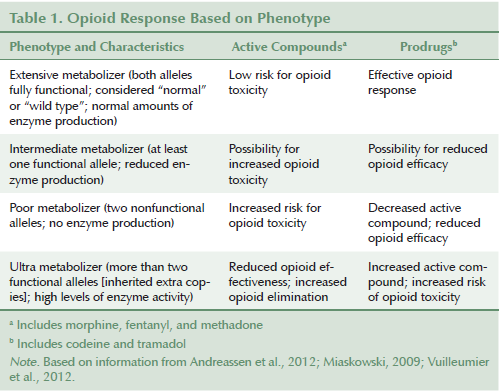
Codeine, a prodrug, must be converted by CYP2D6 to morphine, which is responsible for analgesia (Vuilleumier et al., 2012). After codeine is administered, morphine’s median area under the curve shows significant variability based on phenotype (PMs = 0.5 mcg h/l, EMs = 11 mcg h/l, UMs = 16 mcg h/l) (Fernandez Robles et al., 2012). The PM and UM phenotypes are most predictive of opioid response (Vuilleumier et al., 2012). Tramadol is also metabolized by CYP2D6, with its active metabolite (o-desmethyltramadol) having 200-fold potency over the parent drug (Vuilleumier et al., 2012). This is why PMs who receive tramadol demonstrate significantly lower response rates than EMs (Vuilleumier et al., 2012). UMs using these drugs have a higher potential for drug intoxication and respiratory compromise (Cavallari et al., 2011; Krau, 2013; Miaskowski, 2009; Vuilleumier et al., 2012).
Hydrocodone and oxycodone also have potent active metabolites mediated by CYP2D6 (Vuilleumier et al., 2012). Andreassen et al. (2012) reported that UMs receiving oxycodone receive a 1.5- to 6-fold increase in analgesia over EMs, and that PMs have significantly lower concentrations of active metabolites (oxymorphone and noroxymorphone) than do EMs. Although phenotype has been shown to influence opioid pharmacokinetics, only a few studies validate differences in response. Vuilleumier et al. (2012) reported that analgesia with oxycodone may be weaker in CYP2D6 PMs, whereas Fernandez Robles et al. (2012) noted that oxygen saturation and sedation are more likely to be compromised in UMs than in PMs. Andreassen et al. (2012) explained that, despite measurable differences in oxycodone’s active metabolites, the various phenotypes report no differences in pain intensity, nausea, tiredness, or cognitive function. The conversion of hydrocodone to its active metabolite (dihydrocodone) is, likewise, reduced in PMs; however, phenotype seems to have little effect on a patient’s reported pain intensity (Vuilleumier et al., 2012). The failure to note significant variances in pain response among phenotypes and the scarcity of reported adverse effects (AEs) in UMs may be attributed to hydrocodone’s and oxycodone’s being metabolized by other enzymes, primarily CYP3A4. Table 2 provides a list of CYP enzymes responsible for opioid metabolism.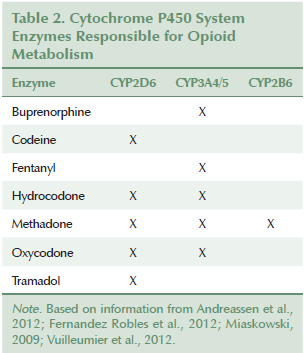
CYP3A: Cytochromic CYP enzymes CYP3A4/5 play a role in the metabolism of fentanyl, methadone, and buprenorphine, as well as hydrocodone and oxycodone. CYP3A4*22 and CYP3A5*3 are two low-functioning alleles that may influence fentanyl concentrations (Barratt et al., 2014), as well as other opioids (Vuilleumier et al., 2012). Individuals with one or more of these low-functioning alleles may be at greater risk for opioid side effects when they receive fentanyl (Miaskowski, 2009). Reports of AEs in PMs receiving hydrocodone or oxycodone are scarce, and AEs have usually been attributed to the coadministration of a food or drug that inhibits production of CYP3A4 (Vuilleumier et al., 2012).
CYP2B6: Methadone is metabolized primarily by CYP2B6, and serum methadone levels can be influenced by an individual’s phenotype (Miaskowski, 2009; Vuilleumier et al., 2012). CYP2B6*5 is a very active allele, and homozygotes will rapidly metabolize methadone (Dobrinas et al., 2013) with low risk of toxicity. CYP2B6*4, CYP2B6*6, CYP2B6*9, and CYP2B6*11 are associated with decreased 2B6 expression (Dobrinas et al., 2013). Individuals with any combination of these four alleles could be at risk for oversedation and respiratory depression.
Drug Target
The receptor is the cellular component that interacts with a drug. The strength of a drug’s response is determined by the configuration of the receptor and the number of receptors available to the drug (Ma & Lu, 2011). Polymorphisms can influence the shape and number of drug receptors, affecting the intensity of response.
The mu opioid receptor gene (OPRM1) has been studied in an effort to explain variant response to opioid therapy. A guanine (G) for adenine (A) substitution (A118G) will replace asparagine with aspartic acid at codon 40, which may affect receptor configuration, influencing the affinity of the opioid to the mu receptor (Miaskowski, 2009; Vuilleumier et al., 2012). This polymorphism may also affect ribonucleic acid expression, influencing the number of mu receptors available to the drug (Vuilleumier et al., 2012). Carriers of this polymorphism (either A/G heterozygotes or G/G homozygotes) experience less than half of the potency of morphine’s active metabolite (M6G) as compared to A/A homozygotes (Miaskowski, 2009), and they require higher opioid doses. Boswell et al. (2013) found that 118A homozygotes have consistently better pain relief with hydrocodone than A/G or G/G genotypes, which can be attributed to higher levels of the active metabolite (hydromorphone). The correlation of pain relief to A/G and G/G was less consistent; these genotypes are more likely to experience opioid-related side effects (Boswell et al., 2013). This A118G substitution has an allelic frequency of about 10%–30% in Caucasians, with a higher prevalence in Asians and a lower prevalence in African Americans (Vuilleumier et al., 2012).
Implications for Nursing and Conclusions
Although a number of polymorphisms influencing opioid therapy have been cited in this article, many of those studies were small and conducted in the perioperative period. How these findings will relate to those with chronic or cancer pain has yet to be determined. Pain is a complicated process that cannot be relegated to a few variant alleles, and pharmacogenomics is just one consideration in variable drug response. Age, comorbidities, drug interactions, and declining organ function can all influence the pharmacokinetics of opioids and the potential for AEs.
Nurses should include ethnicity and family drug history in patients’ pain assessments. Although ethnicity cannot predict the phenotype of patients, certain ethnicities may be more likely to experience AEs from opioid therapies. Nurses must ask patients if any family members have ever had an AE from opioid therapy. Other ethnicities may be at greater risk for opioid failure. Instead of labeling patients as “drug-seeking” or “addicted,” nurses should ask patients if they have failed to respond to opioids in the past or if a family member has failed to respond to a given opioid.
Nurses should know that certain drugs and foods have the potential to alter the production and function of metabolizing enzymes, which can interfere with metabolism of opioids and other drugs. CYP2D6 production is inhibited by many selective serotonin reuptake inhibitors, beta blockers, and tyrosine kinase inhibitors. Coadministration of an opioid with any of these drugs may reduce an EM phenotype to an IM phenotype. CYP3A4 is also inhibited by a number of drugs, including cyclosporine, cimetidine, fluconazole, ketoconazole, norfloxacin, prednisone, and telithromycin (“Cytochrome p450 Drug Interactions,” 2006). Some grapefruit products can inhibit CYP3A4 production. Coadministration of these products with hydrocodone, oxycodone, or tramadol may increase the risk of opioid toxicity. Patients receiving these combinations should be closely monitored for mental status changes or signs of respiratory compromise.
Despite the identification of polymorphisms associated with variable drug response, insufficient evidence exists to suggest that widespread genetic testing will change the efficacy of opioid therapy. The U.S. Food and Drug Administration (2015) identified specific recommendations for only two mu receptor agonists: codeine and tramadol. Administration of codeine to nursing mothers is no longer considered safe practice; the neonate may accumulate toxic levels of morphine if the neonate or its mother happens to be an EM or UM. This scenario has been responsible for serious toxicities and even death (Cavallari et al., 2011; Krau, 2013; Vuilleumier et al., 2012). Concern also exists regarding the administration of o-desmethyltramadol when an individual with the EM or UM phenotype is prescribed tramadol. Although genetic testing is not mandated nor recommended at this time, a provider may want to make considerations for a family history of toxicity from codeine or tramadol therapy or for those of Saudi Arabian or Ethiopian ancestry. Considerations for therapy may include initiating codeine or tramadol at lower doses and monitoring high-risk populations for signs of opioid toxicity.
Whether genetic testing will ever direct all opioid therapy remains to be seen. However, genotyping will likely be a consideration for a number of drugs, particularly for high-risk populations. Many of the allelic variations highlighted in this article may soon help to tailor the opioid choice and its dose to each individual’s genotype, maximizing response to therapy and minimizing risk of AEs, all with the hope of moving clinicians and their patients closer to the promise of personalized medicine.
References
Andreassen, T.N., Eftedal, I., Klepstad, P., Davies, A., Bjordal, K., Lundström, S.,. . . Dale, O. (2012). Do CYP2D6 genotypes reflect oxycodone requirements for cancer patients treated for cancer pain? A cross-sectional multicentre study. European Journal of Clinical Pharmacology, 68, 55–64. doi:10.1007/s00228-011-1093-5
Barratt, D.T., Bandak, B., Klepstad, P., Dale, O., Kaasa, S., Christrup, L.L., . . . Somogyi, A.A. (2014). Genetic, pathological and physiological determinants of transdermal fentanyl pharmacokinetics in 620 cancer patients of the EPOS study. Pharmacogenetics and Genomics, 24, 185–194. doi:10.1097/FPC.0000000000000032
Boswell, M.V., Stauble, M.E., Loyd, G.E., Langman, L., Ramey-Hartung, B., Baumgartner, R.N., . . . Jortani, S.A. (2013). The role of hydromorphone and OPRM1 in postoperative pain relief with hydrocodone. Pain Physician, 16, E227–E235.
Cavallari, L.H., Jeong, H., & Bress, A. (2011). Role of cytochrome P450 genotype in the steps toward personalized drug therapy. Pharmacogenomics and Personalized Medicine, 4, 123–136. doi:10.2147/PGPM.S15497
Cytochrome p450 drug interactions. (2006). Pharmacist’s Letter/Prescriber’s Letter, 22, 220233.
Dobrinas, M., Crettol, S., Oneda, B., Lahyani, R., Rotger, M., Choong, E., . . . Eap, C.B. (2013). Contribution of CYP2B6 alleles in explaining extreme (S)-methadone plasma levels: A CYP2B6 gene resequencing study. Pharmacogenetics and Genomics, 23, 84–93. doi:10.1097/FPC.0b013e32835cb2e2
Eggert, J., & Howe, L. (2010). Pharmacogenomics. In A. Masny, J. Jenkins, & K. Calzone (Eds.), Genetics and genomics in oncology nursing practice (pp. 199–215). Pittsburgh, PA: Oncology Nursing Society.
Fernandez Robles, C.R., Degnan, M., & Candiotti, K.A. (2012). Pain and genetics. Current Opinion in Anaesthesiology, 25, 444–449. doi:10.1097/ACO.0b013e3283556228
Human Cytochrome P450 (CYP) Allele Nomenclature Committee. (2014). CYP2D6 allele nomenclature. Retrieved from http://www.cypalleles.ki.se/cyp2d6.htm
Krau, S.D. (2013). Cytochrome p450, part 1: What nurses really need to know. Nursing Clinics of North America, 48, 671–680. doi:10.1016/j.cnur.2013.09.002
Ma, Q., & Lu, A.Y. (2011). Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacological Reviews, 63, 437–459. doi:10.1124/pr.110.003533
Miaskowski, C. (2009). Understanding the genetic determinants of pain and pain management. Seminars in Oncology Nursing, 25, S1–S7. doi:10.1016/j.soncn.2009.03.012
U.S. Food and Drug Administration. (2015). Table of pharmacogenomic biomarkers in drug labeling. Retrieved from http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics…
Vuilleumier, P.H., Stamer, U.M., & Landau, R. (2012). Pharmacogenomic considerations in opioid analgesia. Pharmacogenomics and Personalized Medicine, 5, 73–87. doi:10.2147/PGPM.S23422
About the Author(s)
Marsha Tadje, RN, MS, AOCN®, is an assistant professor in the College of Nursing at the University of Utah in Salt Lake City. No financial relationships to disclose. Tadje can be reached at marsha.tadje@nurs.utah.edu, with copy to editor at ONFEditor@ons.org.

