Consistency of Breast and Arm Symptoms During the First Two Years After Breast Cancer Surgery
Purpose/Objectives: To examine the severity and development of breast and arm symptoms separately during the two years following breast cancer surgery, and to examine whether previously defined predictors of arm symptoms are associated with breast symptoms.
Design: Prospective cohort study with two-year follow-up.
Setting: Three institutions in the Stockholm, Sweden, region.
Sample: 645 women, aged 20–63 years, enrolled within 12 weeks of surgery for primary breast cancer.
Methods: Baseline register and questionnaire data with five follow-ups were submitted to descriptive, inferential, and logistic regression analysis.
Main Research Variables: Severity of breast and arm symptoms measured by the European Organisation for Research and Treatment of Cancer breast cancer–specific quality-of-life questionnaire.
Findings: Most participants had undergone breast-conserving surgery and sentinel lymph node dissection, and were scheduled for postoperative radiation therapy. Overall mean levels of breast and arm symptoms were low, but with large individual variations. At all six time points, the mean levels of breast symptoms were significantly higher than those of arm symptoms. Overall, the mean level of both types of symptoms decreased during follow-up. A body mass index (BMI) of 25 or greater and breast symptoms at eight months were associated with having breast symptoms at two years. Arm symptoms at baseline and at eight months, and radiation therapy and a BMI of 25 or greater were associated with having arm symptoms at two years.
Conclusions: Breast symptoms show different patterns of change and are not associated with the same factors as arm symptoms.
Implications for Nursing: For nurses monitoring women treated for breast cancer, the results of this study provide knowledge regarding the importance of early symptom identification and long-term symptoms after treatment.
Jump to a section
The long-term consequences of breast cancer (BC)—the most common type of cancer among women (Ferlay et al., 2010)—and its treatments have attracted research interest as survival rates increase (Allen, Savadatti, & Levy, 2009; Champion et al., 2013; McCarthy, 2004; Rosedale, 2009). Long-lasting symptoms in the breast, arm, or axilla region from breast or axillary surgery (e.g., breast-conserving surgery, mastectomy, axillary dissection, sentinel lymph node dissection) and radiation therapy (RT) affect as many as 50% of women in the Nordic countries (Ewertz & Jensen, 2011).
Symptoms in the arm and axilla region are well studied (Albert et al., 2006; Baron, Fey, Borgen, & Van Zee, 2004; Coen, Taghian, Kachnic, Assaad, & Powell, 2003; Hack et al., 2010; Lee, Kilbreath, Refshauge, Herbert, & Beith, 2008; Taira et al., 2011; Verbeek, Spelten, Kammeijer, & Sprangers, 2003), and they have short- and long-term consequences for quality of life and well-being (Carlsen, Harling, Pedersen, Christensen, & Osler, 2013; Engel, Kerr, Schlesinger-Raab, Sauer, & Holzel, 2003; Groeneveld, de Boer, & Frings-Dresen, 2013; Noeres et al., 2013; Reme et al., 2012). Research has shown late consequences of more extensive surgery after completion of adjuvant therapy, related to the type of breast surgery (Ahn et al., 2009; Lindqvist, Stenbeck, & Diderichsen, 2005; Mujahid et al., 2010) and the type of axillary surgery (De Gournay et al., 2013; Johnsson et al., 2009; Lindqvist et al., 2005). Other predictors of arm or shoulder symptoms are younger age (Liljegren & Holmberg, 1997; Yap et al., 2003), having had axillary radiation (Hack et al., 2010; Yap et al., 2003), having had more lymph nodes dissected (Albert et al., 2006; Hack et al., 2010; Liljegren & Holmberg, 1997), and having a higher body mass index (BMI) (Hack et al., 2010; Levy et al., 2012). Early self-reported impairment of arm functioning is also a predictor of late effects of lymph node dissection (Albert et al., 2006).
Less studied are the incidence, frequency, and development of breast symptoms, such as pain, swelling, oversensitivity, and skin problems, caused by surgery and other treatments. Some studies include data on symptoms from the arm and the breast region (Lee, Kilbreath, Refshauge, Herbert, et al., 2008); however, no distinction was made between symptoms from the arm and symptoms from the breast. For example, a swollen arm often includes swelling in the breast region (Taira et al., 2011). In one of the few studies where breast symptoms were studied independently of arm symptoms, Land et al. (2010) concluded that increased morbidity from axillary lymph node dissection is not limited to the arm; rather, breast symptoms increase in severity more after axillary dissection than after sentinel lymph node biopsy, and the difference does not diminish over time.
Taira et al. (2011) showed that a higher percentage of women treated with breast-conserving surgery had symptoms from the breast and arm two years after surgery than those who had undergone mastectomy. As many as 26% of the women treated with breast-conserving surgery experienced severe symptoms such as sensory loss; 16%–17% experienced tightness, tenderness, or discomfort in the arm or breast; and another 15% had lymphedema in the arm. Arndt, Stegmaier, Ziegler, and Brenner (2006) showed that 24% of women treated for BC experienced clinically significant impairments from breast symptoms one year after diagnosis. Lee, Kilbreath, Refshauge, Pendlebury, et al. (2008) showed, in a small sample, that breast symptoms increased in severity from baseline to completion of RT, but returned to baseline levels at the seven-month follow-up. Support for these results also comes from King, Kenny, Shiell, Hall, and Boyages (2000), who found a significant decrease in breast symptom severity during the first year after surgery.
Although the severity of breast and arm symptoms may decrease during the first year after surgery, other studies (Janz et al., 2007) have found that breast symptoms, on average, are more frequent than arm symptoms and are important predictors of decreased body image and emotional functioning. In a previous study of arm and breast morbidity shortly after surgery (Wennman-Larsen, Alexanderson, Olsson, Nilsson, & Petersson, 2013), breast symptoms were more frequently reported than arm symptoms and were more strongly associated with being on sick leave even after controlling for type of breast and axillary surgery.
Although the development and predictors of long-term symptoms in the arm and axilla region after surgery or RT are well studied, the number of studies specifically targeting the incidence, frequency, and development of breast symptoms after surgery is small. The aim of the current study was twofold: (a) to examine the severity and development of breast and arm symptoms separately during the first two years after BC surgery and (b) to examine whether previously well-known predictors of arm symptoms (e.g., age, RT, number of lymph nodes removed, BMI) also are associated with breast symptoms.
The authors developed three hypotheses about the study results. First, the authors believed breast and arm symptoms would become less severe over time, on average, for a majority of the women, with a subgroup of women suffering from long-term symptoms. Second, the authors anticipated higher levels of breast symptoms, on average, than those of arm symptoms within two years after BC surgery. Finally, the authors hypothesized that lower age, more extensive breast or axillary surgery, postoperative RT, higher number of lymph nodes dissected, and a BMI of 25 or greater—all of which have previously been shown to increase the odds of having long-term arm symptoms—would be associated with having long-term breast symptoms.
Methods
This prospective cohort study, with a two-year follow-up, was conducted within the frame of a larger project regarding working-aged women’s life situation and return to work after BC surgery.
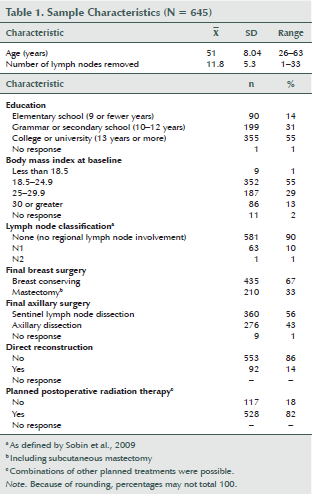
Women who had undergone primary BC surgery at one of three hospitals in the Stockholm, Sweden, area, lived in Stockholm County, were aged 20–63 years, were literate in Swedish, and responded to the baseline questionnaire within 12 weeks of primary BC surgery were consecutively included. Women were excluded if they had known distant metastasis, presurgical chemotherapy, or a previous BC diagnosis. In total, 971 women met the inclusion criteria, but 48 (5%) of those were not evaluated because of administrative errors. The remaining 923 were informed about the study at their first consultation post-BC surgery regarding further treatment decisions (Petersson, Wennman-Larsen, Nilsson, Olsson, & Alexanderson, 2011). Of these, 725 (79%) returned the baseline questionnaire. The 645 women who responded within 12 weeks of primary BC surgery were included in the study (mean response time = 6.3 weeks; SD = 2.8; range = 1–12). The 12 weeks postsurgery criterion was set to limit the time window from surgery. A total of 497 women responded at all six measurement points.
Data Collection
Information about completed breast and axillary surgery and planned RT were obtained for each individual from the Swedish National Quality Register for BC. The clinical population-based registry includes tumor-specific data, surgery data, and planned adjuvant treatment information on all women with BC in Sweden (Regionalt Cancercentrum Stockholm Gotland, 2008). Data on breast and arm symptoms, as well as on other patient information, were obtained from well-validated questionnaires administrated at baseline and after 4, 8, 12, 18, and 24 months.
The women received verbal and written information about the study, stating that participation was voluntary, that their information was confidential, and that they could withdraw at any time. The Regional Ethical Review Board in Stockholm approved the study, and informed consent was obtained from all participants.
Measures
Breast symptoms: Breast symptoms were measured using four self-rated items from the breast symptom scale from the European Organisation for Research and Treatment of Cancer breast cancer–specific quality-of-life questionnaire (EORTC QLQ-BR23) (Sprangers et al., 1996): pain in or around operated breast, swelling in or around operated breast, oversensitivity in area around operated breast, and skin symptoms in or around operated breast during the past week. Participants could choose from four response options: “not at all,” “little,” “quite a bit,” and “very much.” The raw scores were transformed into a 0–100 scale, with high scores indicating more severe symptoms (EORTC, 2001). The responses were summed and divided by number of items, creating an average summated scale for breast symptoms based on a minimum of three responses to items at each of the six measurement points. The Cronbach alpha ranged from 0.71–0.77, and mean inter-item correlation ranged from 0.37–0.45 at the different time points. For the logistic regression analyses, the scale was dichotomized at the 75th percentile at baseline (41.67) (X = 30, SD = 20, range = 0–100) (Rose, Koshman, Spreng, & Sheldon, 1999), meaning that any value greater than 41.67 indicated the presence of breast symptoms.
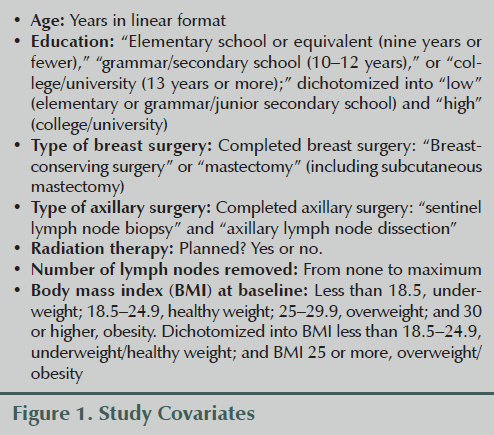
Arm symptoms: Arm symptoms were measured using three items from the EORTC QLQ-BR23 breast symptom scale (Sprangers et al., 1996): pain in arm or shoulder on operated side, swelling arm or hand, and difficulty raising arm or moving it sideways, with the same response options described earlier. An average summated scale was created for arm symptoms, just as it was for breast symptoms. The Cronbach alpha ranged from 0.71–0.77, and mean inter-item correlation ranged from 0.45–0.54 at the different time points. The scale was dichotomized at the 75th percentile at baseline (33.33) (X = 20, SD = 20, range = 0–100) (Rose et al., 1999), meaning that any value greater than 33.33 indicated the presence of arm symptoms. For measures of covariates, see Figure 1.
Statistical Analysis
The authors performed dropout analyses using t tests or chi-square analyses—depending on the level of data—and conducted descriptive analyses regarding proportions and/or level of symptoms using medians, means, and standard deviations. Changes in breast and arm symptoms during the two-year follow-up period were calculated for the 497 women who had responded at all six time points concerning their breast and arm symptoms in a General Linear Model (GLM), repeated-measures analysis of variance (ANOVA) with Greenhouse-Geisser corrections for nonsphericity. Mean differences between breast and arm symptoms at each time point were calculated using paired samples t tests.
Unadjusted and adjusted odds ratios (OR) with 95% confidence intervals (CI) for the probability of having breast or arm symptoms (separately) at two-year follow-up were estimated by logistic regression. To examine the contributions of the covariates for having breast symptoms, each significant variable from the unadjusted analyses was included with breast symptoms at baseline (Model 1). In Model 2, BMI at baseline was entered with breast symptoms at eight months, and in the final model (Model 3), the unadjusted significant variables and breast symptoms at baseline and at eight months were entered simultaneously.
To examine the covariates’ contribution to having arm symptoms, each significant unadjusted variable was included with arm symptoms at baseline (Model 1). In Model 2, significant variables from Model 1 were adjusted for arm symptoms at baseline. In Model 3, significant variables from Model 1 were adjusted for arm symptoms at eight months, and in the final model (Model 4), the significant variables from Model 1 and arm symptoms at baseline and at eight months were entered simultaneously. SPSS®, version 21, was used for all the analyses, and the level of significance was set at p = 0.05.
Drop-Out Analysis
No significant differences in age, type of axillary surgery, type of breast surgery, or planned RT were found between the women who agreed to participate and those who declined participation or were missed because of administrative errors. In addition, no differences in axillary surgery, breast surgery, and planned RT (data not shown) existed between the women who did not respond to the baseline questionnaire within 12 weeks of surgery and the study sample.
Results
At baseline, the mean age of the women in the cohort was 51 years, and more than half had a college or university education. Most had undergone breast-conserving surgery and/or sentinel lymph node dissection, about 33% had had a mastectomy, and 43% underwent axillary dissection (see Table 1). Fourteen percent had undergone immediate reconstruction surgery and 42% had a BMI of 25 or greater; 90% had no lymph node metastases, and 82% were scheduled for postoperative RT.
Severity and Development of Breast and Arm Symptoms
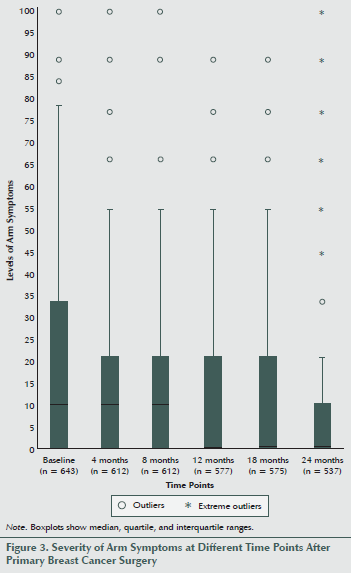
Most women experienced low levels of breast as well as arm symptoms (see Figures 2 and 3). However, at all six time points, the mean levels of breast symptoms were significantly higher than the mean levels of arm symptoms (data not shown, p < 0.009), with the largest difference in means at one year after surgery (9.78 compared with 5.03).
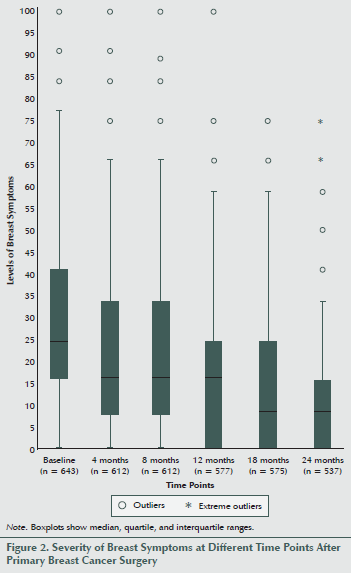
Regarding the levels of breast symptoms among the total sample at all six time points, the median ranged between 25 at baseline and 8.3 at 18 and 24 months. Large variations existed in symptom levels between individuals, and some women experienced severe symptoms for as many as two years after BC surgery, while most women experienced no symptoms.
A repeated-measures ANOVA revealed that the mean levels of breast symptoms for the cohort decreased significantly during the follow-up period (F3.9, 1923.9 = 115.2, p < 0.001) (see Figure 4).
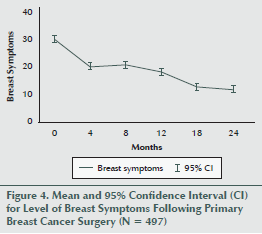
The mean levels of breast symptoms were significantly lower at four months than at baseline (F 1, 496 = 110.3, p < 0.001), with no significant difference between four and eight months (F 1, 496 = 2.3, p = 0.131). Levels were also lower at 12 months than at 8 months (F 1, 496 = 22, p < 0.001), at 18 months than at 12 months (F 1, 496 = 29.3, p < 0.001), and at 24 months than at 18 months (F 1, 496 = 8.7, p = 0.003).
The median level of arm symptoms ranged between 11.1 at baseline and 0 at 12, 18, and 24 months. As in the case of breast symptoms, large variations existed between individuals, and some women experienced severe symptoms up to two years after surgery, while most had no symptoms.
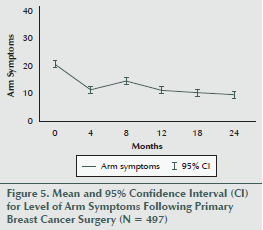
The mean levels of arm symptoms also decreased significantly during the two years following BC surgery (F 4.45, 2207.35 = 42, p < 0.001) (see Figure 5). During follow-up, symptom levels decreased between baseline and 4 months (F 1, 496 = 11.6, p < 0.001), increased between 4 and 8 months (F 1, 496 = 34.7, p < 0.001), decreased between 8 and 12 months (F 1, 496 = 19.2, p < 0.001), and leveled out with no significant changes between 12 and 18 months (F 1, 496 = 0.8, p = 0.363) and 18 and 24 months (F 1, 496 = 1.1, p = 0.286).
Factors Associated With Long-Term Breast or Arm Symptoms
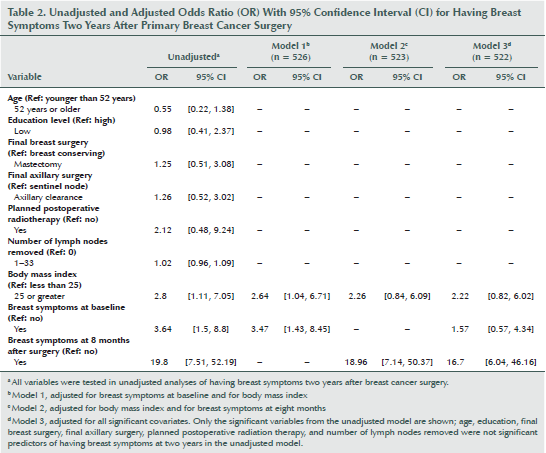
The factors associated with having long-term breast symptoms were not consistent with those associated with having long-term arm symptoms. In the unadjusted analyses, those with a BMI of 25 or greater and breast symptoms at baseline and at eight months had higher ORs for having breast symptoms two years after BC surgery (see Table 2). Adjustment for breast symptoms at baseline attenuated the OR somewhat (OR = 2.64; 95% CI [1.04, 6.71]) (Model 1), and the OR did not remain significant after adjustment for breast symptoms at eight months (Model 2). In Model 3, after adjusting for all covariates, the only factor associated with having breast symptoms after two years was having breast symptoms at eight months (OR = 16.7; 95% CI [6.04, 46.16]).
Regarding arm symptoms in the unadjusted analyses, those having had RT, axillary dissection, a BMI of 25 or greater at baseline, and/or more lymph nodes removed had a higher OR for having arm symptoms two years after BC surgery (see Table 3). In Model 1, including all variables significant from the unadjusted analyses, but without adjusting for previous arm symptoms, a BMI of 25 or greater and planned postoperative RT were associated with arm symptoms at two years (OR = 4.68; 95% CI [1.1, 19.94] and OR = 2; 95% CI [1.1, 3.77], respectively). The association remained when adjusting for arm symptoms at baseline (Model 2), but not after adjustment for arm symptoms at eight months (Model 3). When adjusting for all the covariates (Model 4), having arm symptoms at baseline (OR = 5.4; 95% CI [2.5, 11.66]) and at eight months (OR = 14.78; 95% CI [6.86, 31.86]) were associated with having arm symptoms at two years.
[[{"type":"media","view_mode":"media_original","fid":"19666","attributes":{"alt":"","class":"media-image","height":"351","typeof":"foaf:Image","width":"732"}}]]
Discussion
In this prospective cohort study of women who had BC surgery, the results suggest that breast symptoms should be considered separately from arm symptoms because different factors were associated with having long-term symptoms. Even if the mean levels of both breast and arm symptoms were low, large individual variations existed, and the mean levels of breast symptoms were higher than those of arm symptoms. Having symptoms at eight months and having a BMI of 25 or greater were both associated with having long-term arm and breast symptoms two years after BC surgery. However, regarding arm symptoms, having symptoms shortly after surgery and planning for postoperative RT were also associated with having symptoms at the two-year follow-up.
Severity of Breast and Arm Symptoms
Supporting the authors’ first hypothesis, the mean levels of breast and arm symptoms decreased over the two-year period; however, the patterns for breast and arm symptoms were different. In addition, although the mean symptom levels were generally low, some women had severe symptoms at eight months and at two years after surgery. In line with the authors’ second hypothesis, the mean levels of breast symptoms were significantly higher than those of arm symptoms at all time points. Although arm symptoms are one of the most studied and reported sets of symptoms after BC (Coen et al., 2003; Hack et al., 2010; Lee, Kilbreath, Refshauge, Herbert, et al., 2008; Taira et al., 2011), breast symptoms are more frequently reported by women during the first year after surgery (Janz et al., 2007). The higher technical sophistication of surgery (Krag et al., 2007) and the lower dosage and more precisely directed RT (Hopwood et al., 2010; Versmessen et al., 2012) have minimized the arm side effects of surgery and RT. However, previous research focused less on breast symptoms, and greater attention should be paid to them in future studies.
Symptom-Associated Factors at the End of the Follow-Up Period
The hypothesis that the factors associated with breast symptoms would be the same as those of arm symptoms (Ahn et al., 2009; Hack et al., 2010; Johnsson et al., 2009; Levy et al., 2012; Liljegren & Holmberg, 1997; Lindqvist et al., 2005; Mujahid et al., 2010; Yap et al., 2003) was not supported. Having a BMI of 25 or greater and early breast symptoms were the only factors associated with breast symptoms at two years. In addition, having a high BMI at baseline and planned RT were the only two predictors of arm symptoms, which is supported by the findings of previous research (Hack et al., 2010; Karki, Simonen, Malkia, & Selfe, 2005; Levy et al., 2012; Nesvold, Fossa, Holm, Naume, & Dahl, 2010; Yap et al., 2003). As stated in the literature, the influence of BMI warrants additional investigation because the mechanisms are unknown (Levy et al., 2012). Such mechanisms might be investigated on the basis of the current study’s as well as others’ results (Hack et al., 2010; Karki et al., 2005; Levy et al., 2012; Nesvold et al., 2010; Yap et al., 2003), and the associations of high BMI and long-term breast and arm symptoms should be explored further in this patient group. In the current study, 42% of the women were overweight or obese at baseline. That statistic is important to note because Karki et al. (2005) showed that women tend to gain rather than lose weight after BC surgery. Being overweight may make exercise, with a preventive effect on development of arm symptoms, difficult (McNeely et al., 2010).
Even if previous results are lacking, it was surprising that neither type of surgery nor RT was associated with having breast symptoms. As mentioned previously, past research has shown that axillary radiation (Hack et al., 2010) and RT directed at the breast (Yap et al., 2003) predict arm symptoms, particularly lymphedema, arm swelling, and shoulder restriction (Lee, Kilbreath, Refshauge, Herbert, et al., 2008). In a study of RT dose regimens, Hopwood et al. (2010) found that 40% of women have marked changes to the breast after RT and 10%–24% have breast symptoms, as measured by the items in the BR23 breast symptom scale—irrespective of dose. The results regarding changes over time—which show a temporary increase in breast and arm symptoms shortly after surgery, followed, in most women, by a decrease during the first year after surgery—are similar to results from Lee, Kilbreath, Refshauge, Pendlebury, et al. (2008) and Browall et al. (2008). That similarity implies that even if the type of breast or axillary surgery or RT are not significantly associated with breast symptoms two years after surgery, detectable short-term effects of surgery and RT do exist. However, in the current study, the mean levels of breast symptoms at baseline were higher than in Lee, Kilbreath, Refshauge, Pendlebury, et al.’s (2008) study, which may be a result of the current study’s baseline measurement being closer to the time of surgery.
In general, however, comparing the prevalence, incidence, and mean levels of breast and arm symptoms between studies is problematic because assessment measures, times of measurement, and sample characteristics vary widely. In a review by Lee, Kilbreath, Refshauge, Herbert, et al. (2008), less than 1%–67% of the women reported shoulder restriction, 0%–34% reported lymphedema, 9%–68% reported shoulder or arm pain, and 9%–28% reported weakness, sometimes many years after BC surgery and RT. One possible limitation of the current study is the use of only self-rated measures of arm and breast symptoms. Another possible limitation concerns the relevance of the chosen questionnaires. Although the EORTC QLQ-BR23 (Sprangers et al., 1996), with its breast and arm symptom scales, is one of the most used and well-validated measures in the field, it was developed in the mid-1990s. The consequences of surgery and RT have changed with the development of treatments, such as sentinel lymph node dissection, that have reduced the incidence of lymphedema. Such developments, of course, may have implications for the validity of the arm symptoms scale in particular. In the arm symptoms scale, lymphedema is calculated together with pain in the arm or shoulder and difficulties in raising the arm or moving it sideways. Although lymphedema often develops later, the pain may be most severe shortly after surgery or RT. Such lack of overlap between symptoms has been demonstrated previously (Thomas-Maclean et al., 2008).
Strengths and Limitations
The strengths of this study include the large sample size, the high response rate, the long follow-up, the use of six measures over time, data that was first collected soon after BC surgery, and validated measures for exposure and outcome. Limitations include that some women dropped out during the follow-up, a common phenomenon in cohort studies based on surveys (Carter, Imlach-Gunasekara, McKenzie, & Blakely, 2012). One consequence of the survey-based study design may be selection bias in the dropout during follow-up because participants with the greatest problems may not report them, or those without problems may choose not to respond. Another limitation is that, as in all surveys, respondents might have interpreted the questions differently. However, the results of this study may have clinical relevance because they show that predictions of persistent symptoms are possible even early after surgery and irrespective of type of treatment.
Implications for Nursing Practice
Nurse-led follow-up is an important contribution to the care of the growing numbers of survivors, particularly for routine monitoring after BC (Bessen et al., 2014; Koinberg, Fridlund, Engholm, & Holmberg, 2004; Koinberg, Holmberg, & Fridlund, 2002; van Hezewijk, van den Akker, van de Velde, Scholten, & Hille, 2012). Specialist nurse-led follow-up care provides as much patient satisfaction and safety as that of specialist physicians (Koinberg et al., 2002, 2004), and has proven to be preferable to long-term follow-up by general practitioners (Bessen et al., 2014). Nurses play an increasingly important role in the monitoring of women who have undergone treatment for BC, so the results of this study may provide important knowledge regarding the importance of early symptom identification and the long-term side effects of treatment. In addition, the current study found that overweight or obese women may have higher odds for experiencing long-term breast or arm symptoms. Future studies need to find ways to support overweight and obese patients in avoiding breast or arm symptoms.
[[{"type":"media","view_mode":"media_original","fid":"19671","attributes":{"alt":"","class":"media-image","height":"235","typeof":"foaf:Image","width":"366"}}]]
Conclusion
Breast symptoms show different patterns of change and are not associated with the same previously defined predictors as arm symptoms. Having breast symptoms early after BC surgery and a BMI of 25 or greater indicate a risk of long-term symptoms, irrespective of type of treatment. Having arm symptoms early after surgery and a BMI of 25 or greater indicate a risk of long-term symptoms together with planned, postoperative RT.
References
Ahn, E., Cho, J., Shin, D.W., Park, B.W., Ahn, S.H., Noh, D.Y., . . . Yun, Y.H. (2009). Impact of breast cancer diagnosis and treatment on work-related life and factors affecting them. Breast Cancer Research and Treatment, 116, 609–616.
Albert, U.S., Koller, M., Kopp, I., Lorenz, W., Schulz, K.D., & Wagner, U. (2006). Early self-reported impairments in arm functioning of primary breast cancer patients predict late side effects of axillary lymph node dissection: Results from a population-based cohort study. Breast Cancer Research and Treatment, 100, 285–292. doi:10.1007/s10549-006-9247-3
Allen, J.D., Savadatti, S., & Levy, A.G. (2009). The transition from breast cancer “patient” to “survivor.” Psycho-Oncology, 18(1), 71–78. doi:10.1002/pon.1380
Arndt, V., Stegmaier, C., Ziegler, H., & Brenner, H. (2006). A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer, 107, 2496–2503.
Baron, R.H., Fey, J.V., Borgen, P.I., & Van Zee, K.J. (2004). Eighteen sensations after breast cancer surgery: A two-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Oncology Nursing Forum, 31, 691–698. doi:10.1188/04.ONF.691-698
Bessen, T., Chen, G., Street, J., Eliott, J., Karnon, J., Keefe, D., & Ratcliffe, J. (2014). What sort of follow-up services would Australian breast cancer survivors prefer if we could no longer offer long-term specialist-based care? A discrete choice experiment. British Journal of Cancer, 110, 859–867. doi:10.1038/bjc.2013.800
Browall, M., Ahlberg, K., Karlsson, P., Danielson, E., Persson, L.O., & Gaston-Johansson, F. (2008). Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. European Journal of Oncology Nursing, 12, 180–189.
Carlsen, K., Harling, H., Pedersen, J., Christensen, K.B., & Osler, M. (2013). The transition between work, sickness absence and pension in a cohort of Danish colorectal cancer survivors. British Medical Journal Open, 3(2), e002259. doi:10.1136/bmjopen-2012-002259
Carter, K.N., Imlach-Gunasekara, F., McKenzie, S.K., & Blakely, T. (2012). Differential loss of participants does not necessarily cause selection bias. Australian and New Zealand Journal of Public Health, 36, 218–222. doi:10.1111/j.1753-6405.2012.00867.x
Champion, V.L., Ziner, K.W., Monahan, P.O., Stump, T.E., Cella, D., Smith, L.G., . . . Sledge, G.W. (2013). Development and psychometric testing of a breast cancer survivor self-efficacy scale [Online exclusive]. Oncology Nursing Forum, 40, E403–E410. doi:10.1188/13.ONF.E403-E410
Coen, J.J., Taghian, A.G., Kachnic, L.A., Assaad, S.I., & Powell, S.N. (2003). Risk of lymphedema after regional nodal irradiation with breast conservation therapy. International Journal of Radiation Oncology, Biology, Physics, 55, 1209–1215.
De Gournay, E., Guyomard, A., Coutant, C., Boulet, S., Arveux, P., Causeret, S., . . . Dabakuyo-Yonli, T.S. (2013). Impact of sentinel node biopsy on long-term quality of life in breast cancer patients. British Journal of Cancer, 109, 2783–2791. doi:10.1038/bjc.2013.658
Engel, J., Kerr, J., Schlesinger-Raab, A., Sauer, H., & Holzel, D. (2003). Axilla surgery severely affects quality of life: Results of a 5-year prospective study in breast cancer patients. Breast Cancer Research and Treatment, 79, 47–57.
European Organisation for Research and Treatment of Cancer. (2001). EORTC-C30 scoring manual (3rd ed.). Brussels, Belgium: Author.
Ewertz, M., & Jensen, A.B. (2011). Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncologica, 50, 187–193. doi:10.3109/0284186X.2010.533190
Ferlay, J., Shin, H.R., Bray, F., Forman, D., Mathers, C., & Parkin, D.M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127, 2893–2917.
Groeneveld, I.F., de Boer, A.G., & Frings-Dresen, M.H. (2013). Physical exercise and return to work: Cancer survivors’ experiences. Journal of Cancer Survivorship, 7, 237–246. doi:10.1007/s11764-012-0264-4
Hack, T.F., Kwan, W.B., Thomas-MacLean, R.L., Towers, A., Miedema, B., Tilley, A., & Chateau, D. (2010). Predictors of arm morbidity following breast cancer surgery. Psycho-oncology, 19, 1205–1210.
Hopwood, P., Haviland, J.S., Sumo, G., Mills, J., Bliss, J.M., & Yarnold, J.R. (2010). Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncology, 11, 231–240.
Janz, N.K., Mujahid, M., Chung, L.K., Lantz, P.M., Hawley, S.T., Morrow, M., . . . Katz, S.J. (2007). Symptom experience and quality of life of women following breast cancer treatment. Journal of Women’s Health, 16, 1348–1361.
Johnsson, A., Fornander, T., Rutqvist, L.E., Vaez, M., Alexanderson, K., & Olsson, M. (2009). Predictors of return to work ten months after primary breast cancer surgery. Acta Oncologica, 48(1), 93–98.
Karki, A., Simonen, R., Malkia, E., & Selfe, J. (2005). Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. Journal of Rehabilitation Medicine, 37, 180–188.
King, M.T., Kenny, P., Shiell, A., Hall, J., & Boyages, J. (2000). Quality of life three months and one year after first treatment for early stage breast cancer: Influence of treatment and patient characteristics. Quality of Life Research, 9, 789–800.
Koinberg, I.L., Fridlund, B., Engholm, G.B., & Holmberg, L. (2004). Nurse-led follow-up on demand or by a physician after breast cancer surgery: A randomised study. European Journal of Oncology Nursing, 8, 109–117. doi:10.1016/j.ejon.2003.12.005
Koinberg, I.L., Holmberg, L., & Fridlund, B. (2002). Breast cancer patients’ satisfaction with a spontaneous system of check-up visits to a specialist nurse. Scandinavian Journal of Caring Sciences, 16, 209–215.
Krag, D.N., Anderson, S.J., Julian, T.B., Brown, A.M., Harlow, S.P., Ashikaga, T., . . . Wolmark, N. (2007). Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncology, 8, 881–888. doi:10.1016/S1470-2045(07)70278-4
Land, S.R., Kopec, J.A., Julian, T.B., Brown, A.M., Anderson, S.J., Krag, D.N., . . . Ganz, P.A. (2010). Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. Journal of Clinical Oncology, 28, 3929–3936. doi:10.1200/JCO.2010.28.2491
Lee, T.S., Kilbreath, S.L., Refshauge, K.M., Herbert, R.D., & Beith, J.M. (2008). Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Research and Treatment, 110(1), 19–37. doi:10.1007/s10549-007-9710-9
Lee, T.S., Kilbreath, S.L., Refshauge, K.M., Pendlebury, S.C., Beith, J.M., & Lee, M.J. (2008). Quality of life of women treated with radiotherapy for breast cancer. Supportive Care in Cancer, 16, 399–405.
Levy, E.W., Pfalzer, L.A., Danoff, J., Springer, B.A., McGarvey, C., Shieh, C.Y., . . . Stout, N.L. (2012). Predictors of functional shoulder recovery at 1 and 12 months after breast cancer surgery. Breast Cancer Research and Treatment, 134(1), 315–324. doi:10.1007/s10549-012-2061-1
Liljegren, G., & Holmberg, L. (1997). Arm morbidity after sector resection and axillary dissection with or without postoperative radiotherapy in breast cancer stage I. Results from a randomised trial. Uppsala-Orebro Breast Cancer Study Group. European Journal of Cancer, 33, 193–199.
Lindqvist, R., Stenbeck, M., & Diderichsen, F. (2005). Does hospital discharge policy influence sick-leave patterns in the case of female breast cancer? Health Policy, 72(1), 65–71. doi:10.1016/j.healthpol.2004.06.003
McCarthy, N.J. (2004). Care of the breast cancer survivor: Increased survival rates present a new set of challenges. Postgraduate Medicine, 116(4), 39–40, 42, 45–46.
McNeely, M.L., Campbell, K., Ospina, M., Rowe, B.H., Dabbs, K., Klassen, T.P., . . . Courneya, K. (2010). Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database of Systematic Reviews, 6, CD005211. doi:10.1002/14651858.CD005211.pub2
Mujahid, M., Janz, N., Hawley, S., Griggs, J., Hamilton, A., & Katz, S. (2010). The impact of sociodemographic, treatment, and work support on missed work after breast cancer diagnosis. Breast Cancer Research and Treatment, 119(1), 213–220. doi:10.1007/s10549-009-0389-y
Nesvold, I.L., Fossa, S.D., Holm, I., Naume, B., & Dahl, A.A. (2010). Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncologica, 49, 347–353. doi:10.3109/02841860903302905
Noeres, D., Park-Simon, T.W., Grabow, J., Sperlich, S., Koch-Gießelmann, H., Jaunzeme, J., & Geyer, S. (2013). Return to work after treatment for primary breast cancer over a 6-year period: Results from a prospective study comparing patients with the general population. Supportive Care in Cancer, 1–9. doi:10.1007/s00520-013-1739-1
Petersson, L.M., Wennman-Larsen, A., Nilsson, M., Olsson, M., & Alexanderson, K. (2011). Work situation and sickness absence in the initial period after breast cancer surgery. Acta Oncologica, 50, 282–288. doi:10.3109/0284186X.2010.533191
Regionalt Cancercentrum Stockholm Gotland. (2008). Bröstcancer: Nationellt kvalitetsregister för bröstcancer [the National Quality Register for Breast Cancer]. Retrieved from http://www.cancercentrum.se/sv/stockholmgotland/VP_register/Brostcancer…
Reme, S., Shaw, W., Steenstra, I., Woiszwillo, M., Pransky, G., & Linton, S. (2012). Distressed, immobilized, or lacking employer support? A sub-classification of acute work-related low back pain. Journal of Occupational Rehabilitation, 22, 541–552. doi:10.1007/s10926-012-9370-4
Rose, M.S., Koshman, M.L., Spreng, S., & Sheldon, R. (1999). Statistical issues encountered in the comparison of health-related quality of life in diseased patients to published general population norms: Problems and solutions. Journal of Clinical Epidemiology, 52, 405–412.
Rosedale, M. (2009). Survivor loneliness of women following breast cancer. Oncology Nursing Forum, 36, 175–183. doi:10.1188/09.ONF.175-183
Sobin, L., Gospodarowicz, M., & Wittekond, C. (2009). TNM classification of malignant tumours (7th ed.) New York, NY: Wiley-Liss.
Sprangers, M.A., Groenvold, M., Arraras, J.I., Franklin, J., te Velde, A., Muller, M., . . . Aaronson, N.K. (1996). The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. Journal of Clinical Oncology, 14, 2756–2768.
Taira, N., Shimozuma, K., Shiroiwa, T., Ohsumi, S., Kuroi, K., Saji, S., . . . Katsumata, N. (2011). Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Research and Treatment, 128, 735–747. doi:10.1007/s10549-011-1631-y
Thomas-Maclean, R.L., Hack, T., Kwan, W., Towers, A., Miedema, B., & Tilley, A. (2008). Arm morbidity and disability after breast cancer: New directions for care. Oncology Nursing Forum, 35, 65–71. doi:10.1188/08.ONF.65-71
van Hezewijk, M., van den Akker, M.E., van de Velde, C.J., Scholten, A.N., & Hille, E.T. (2012). Costs of different follow-up strategies in early breast cancer: A review of the literature. Breast, 21, 693–700. doi:10.1016/j.breast.2012.09.009
Wennman-Larsen, A., Alexanderson, K., Olsson, M., Nilsson, M. I., & Petersson, L.M. (2013). Sickness absence in relation to breast and arm symptoms shortly after breast cancer surgery. Breast, 22, 767–772. doi:10.1016/j.breast.2013.01.006
Verbeek, J., Spelten, E., Kammeijer, M., & Sprangers, M. (2003). Return to work of cancer survivors: A prospective cohort study into the quality of rehabilitation by occupational physicians. Occupational and Environmental Medicine, 60, 352–357.
Versmessen, H., Vinh-Hung, V., Van Parijs, H., Miedema, G., Voordeckers, M., Adriaenssens, N., . . . De Ridder, M. (2012). Health-related quality of life in survivors of stage I-II breast cancer: Randomized trial of post-operative conventional radiotherapy and hypofractionated tomotherapy. BMC Cancer, 12, 495. doi:10.1186/1471-2407-12-495
Yap, K.P., McCready, D.R., Narod, S., Manchul, L.A., Trudeau, M., & Fyles, A. (2003). Factors influencing arm and axillary symptoms after treatment for node negative breast carcinoma. Cancer, 97, 1369–1375. doi:10.1002/cncr.11218
About the Author(s)
Agneta Wennman-Larsen, RN, PhD, is a researcher in the Division of Insurance Medicine and the Department of Clinical Neuroscience at Karolinska Institutet (KI) and an associate professor and senior lecturer at Sophiahemmet University in Stockholm; Lena-Marie Petersson, RN, OCN®, RNT, PhD, is a researcher in the Division of Insurance Medicine and the Department of Clinical Neuroscience at KI; Fredrik Saboonchi, PhD, is an associate professor in the Division of Insurance Medicine and the Department of Clinical Neuroscience at KI and a senior lecturer at the Red Cross University College in Stockholm; Kristina Alexanderson, BSc, PhD, is a professor in the Division of Insurance Medicine and Department of Clinical Neuroscience at KI; and Marjan Vaez, MSc, PhD, is an associate professor in the Division of Insurance Medicine and the Department of Clinical Neuroscience at KI and an epidemiologist at the Centre for Occupational and Environmental Medicine, Stockholm County Council, all in Sweden. This study was supported, in part, by the Swedish Cancer Society; the Swedish Research Council; the Cancer Research Foundation of Radiumhemmet; the Swedish Research Council for Health, Working Life and Welfare; and the Breast Cancer Foundation. Wennman-Larsen can be reached at agneta.wennman-larsen@ki.se, with copy to editor at ONFEditor@ons.org. (Submitted February 2014. Accepted for publication October 2, 2014.)

