Longitudinal Subgrouping of Patients With Cancer Based on Symptom Experiences: An Integrative Review
Problem Identification: The purpose of this integrative review is to identify literature describing (a) subgrouping patients with cancer based on symptom experiences and their patterns of symptom changes over time and (b) methodologies of subgrouping patients with cancer based on symptom experiences.
Literature Search: PubMed®, CINAHL®, and PsycINFO® were searched through January 2019.
Data Evaluation: Studies were appraised for patterns of symptom change over time and methodologic approach using the QualSyst quality rating scale.
Synthesis: 11 studies met inclusion criteria. Clinical variables that influence symptom patterns were diverse. The most common clustering method was latent variable analysis (73%), and all studies collected symptom data prospectively using survey analysis to assess symptoms.
Implications for Practice: The majority of studies (91%) observed that the symptom experience within the group of patients with cancer changed over time. Identifying groups of patients with similar symptom experiences is useful to determine which patients need more intensive symptom management over the trajectory of cancer treatment, which is essential to improve symptoms and quality of life.
Jump to a section
Patients with cancer face a number of severe symptoms, such as pain (Brant et al., 2011), fatigue (Ameringer et al., 2013; Brant et al., 2011), sleep disturbance (Ameringer et al., 2013; Brant et al., 2011), depression (Brant et al., 2011), and nausea (Ameringer et al., 2013). Experiencing these symptoms has been shown to affect functional status and quality of life in people with cancer (Hwang et al., 2003; Kroenke et al., 2013). Symptom management in this population is further complicated by the experience of having multiple co-occurring symptoms, known as symptom clusters (SCs) (Bender et al., 2008; Dodd et al., 2004; Fan et al., 2007). Several studies reported that the occurrence of SCs negatively affects patient outcomes, such as patients’ functional status (Dodd et al., 2001; Doong et al., 2015; Illi et al., 2012; Kim et al., 2012; Lin et al., 2013; Miaskowski et al., 2006, 2007; Rha & Lee, 2017; Ryu et al., 2010), quality of life (Dodd et al., 2010; Fox & Lyon, 2006; Gold et al., 2016; Henoch & Lövgren, 2014; Hwang et al., 2016; Lin et al., 2013; Miaskowski et al., 2006, 2007; Nho et al., 2017; Phligbua et al., 2013; Rha & Lee, 2017; Ryu et al., 2010), or healthcare use (Miaskowski et al., 2017).
A literature review reported the most commonly identified SCs in chronic conditions, including cancer and other rare diseases, as (a) fatigue, pain, depression, and sleep disturbance; (b) nausea and vomiting; and (c) anxiety and depression (Miaskowski et al., 2017). These symptom profiles may vary based on demographic and clinical characteristics and may change based on disease trajectories over time. Groups of patients may share similar experiences of a particular SC (e.g., all mild symptoms, all severe symptoms, or a mixture of both). The National Institute of Nursing Research (2019) set a research priority with the goal of facilitating delivery of tailored and effective symptom management interventions. To achieve this goal, there is a need to identify subgroups of patients based on symptom experiences over time.
Two main methodologic approaches to SC research are (a) identifying groups of interlinked symptoms using an empirical or exploratory approach and (b) identifying subgroups of patients with similar symptom experiences on a selected SC (Miaskowski et al., 2007). Both approaches are important but address different priorities—what symptoms cluster as opposed to who experiences this cluster. Four review articles have examined SCs in patients with cancer (Dong et al., 2014; Fan et al., 2007; Gilbertson-White et al., 2011; Thavarajah et al., 2012). However, to date, most oncology studies on SC have focused on groups of interlinked symptoms (i.e., what symptoms cluster rather than who experiences this cluster) (Fan et al., 2007) and used cross-sectional data rather than long-term symptom trajectories (Thavarajah et al., 2012). Of the limited research on longitudinal SCs, all the published literature has focused on exploring how specific SCs (i.e., pain, fatigue, and sleep disturbance) evolve over time. To the authors’ knowledge, there has been no synthesis of the literature integrating SCs based on patient subgroups, their patterns of symptom change over time, and methodologies of patient subgrouping with similar symptom experiences longitudinally (over three or more time points). Subgrouping patients at risk for developing SCs at the same time allows researchers to understand who may be at increased risk for severe symptoms, guide the selection of appropriate symptom management interventions before symptoms manifest or intensify, and examine the relationship between distinct symptom profiles and patient outcomes.
The purpose of this article is to identify literature describing the following: (a) subgrouping patients with cancer based on symptom experiences and their patterns of symptom changes over time within patient groups and (b) methodologies of longitudinal studies subgrouping patients with similar symptom experiences. Patterns of symptom experiences within different groups of patients can vary based on type of cancer, different treatments, when and how frequently symptoms were evaluated, and which symptoms were assessed. Therefore, the authors will also examine cancer diagnoses and treatments, the number of time points for assessing symptoms, when and how frequently symptoms were evaluated, symptom assessment tools with regard to symptom dimensions and symptoms included for assessment, and approaches to data collection.
Methods
Literature Search and Study Selection
A literature search was conducted according to the methodology proposed by Whittemore and Knafl (2005). PubMed®, CINAHL®, and PsycINFO® were systematically searched from inception through January 2019 using the following keywords or MeSH (Medical Subject Headings) terms: Signs and Symptoms AND cluster* AND neoplasm* AND Time Factors. The following derivatives were used to ensure comprehensiveness: (cluster* OR constellation* OR combination* OR cooccurrence OR group* OR multiple OR concurrent), (cancer OR neoplasm* OR oncolog*), (Trajector* OR longitudinal OR time OR change* OR pattern*). The list of database search terms was developed with the assistance of a health science librarian to ensure a systematic search of the literature.
Eligibility for inclusion in the review included the following:
- Studies with a sample of individuals diagnosed with any cancer and of any age because the focus of this review was on different analytical methods of subgrouping symptom experience of patients with cancer
- Studies that described subgroups of patients with cancer based on their experience with established SCs
- Longitudinal studies that identified subgroups of patients with changes in their symptom experience over three or more time points
Exclusion criteria were as follows:
- Studies that examined whether SC composition in the groups of patients with cancer changed over time
- Studies that described a single symptom or multiple symptoms but did not necessarily consider them in a cluster (a set of co-occurring symptoms that are believed to be related to each other)
- Studies that tested mechanisms for SCs
- Experiences of caregivers of patients with cancer
- Studies published in languages other than English, and with no full text
Quality Assessment and Data Extraction
One author (S.C.) and an undergraduate research assistant used the eligibility criteria to evaluate titles and abstracts independently, and then two authors with cancer symptom expertise (C.C. and S.G.W.) confirmed the selected articles. The second author (C.C.) also read full texts of all the articles that met the criteria for inclusion to ensure agreement.
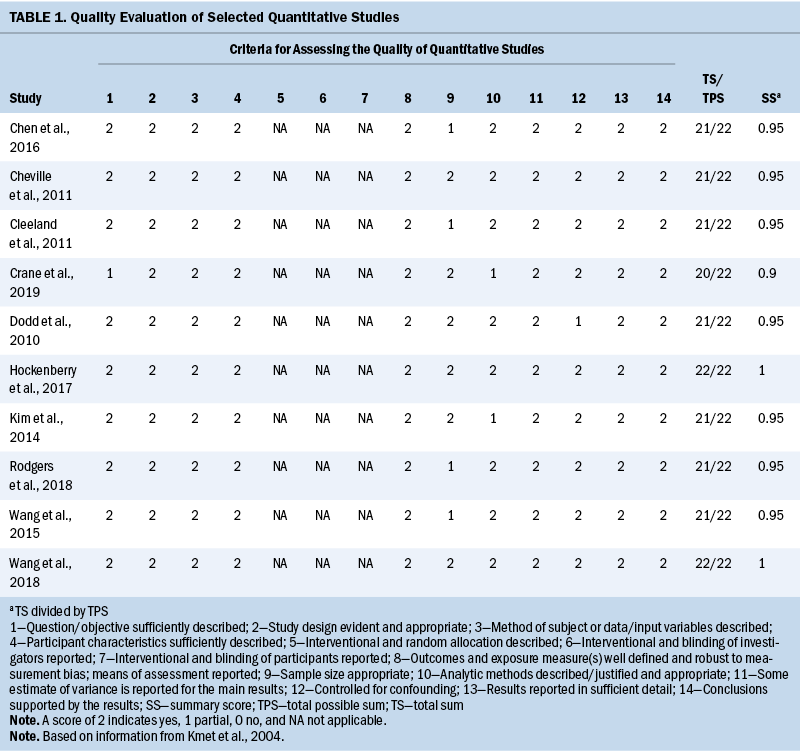
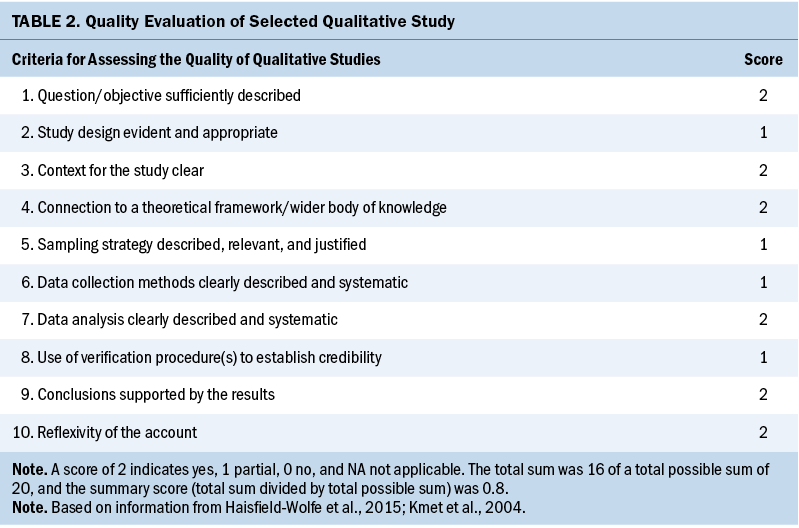
One author (S.C.) assessed studies for quality using 14 criteria (description of objective, study design, method, participants description, randomization, blinding of investigators, blinding of participants, outcome and exposure measurement, sample size, analytic methods, reporting an estimate of variance, controlling confounding, reporting results, conclusions supported by the results) of the QualSyst quality rating scale, which was developed to appraise qualitative and quantitative studies (Kmet et al., 2004). Criteria were individually assessed and scored (2 = yes, 1 = partial, 0 = no, NA = not applicable). For each study included in this review, a total score was calculated as the number of points awarded by the reviewer divided by the total available points (range from 0% to 100%). If the final score of each study was more than 80%, 70%, and 50%, the quality was considered as strong, good, and adequate, respectively, as outlined by Lee et al. (2008) (see Tables 1 and 2).
Data analysis was performed using the five-stage methodology—data reduction, display, comparison, conclusion drawing and verification, and presentation—as described by Whittemore and Knafl (2005). For each study, the authors extracted the following information: the design (the number of time points for assessing symptoms and the timespan between assessments); sample characteristics (including sample size, type of cancer, type of treatments); symptom assessments (including the total number of symptom assessments per study, the most frequently studied symptoms, the most commonly used symptom assessment tools, symptom dimension, prespecified symptom clusters); primary purposes; method(s); key findings; outcomes; and limitations. To describe patterns of symptom changes over time within patient groups, the authors identified the stability of patterns of symptoms within patient subgroups based on cancer diagnosis, treatment, and symptom dimensions. Stability was defined as the changes or transitions between different patient groups of symptom experiences according to groups across time.
Evaluation of Methods Used to Identify Subgroups
From the authors’ expertise in SCs, symptom management, and biostatistics, they developed four criteria (2 = yes, 1 = partial, 0 = no). Scores were then added, resulting in a total score to evaluate the methodologies applied in the selected studies. Interpretability refers to methods that can help researchers understand the results. Reproducibility is the capacity to implement the analytical procedures using other data but the same methods. Flexibility is the ability to adapt to various study designs and diverse types of data, including potentially complex patterns of dependence among variables of interest. Acceptance/popularity refers to how widely used this approach is in the literature.
Results
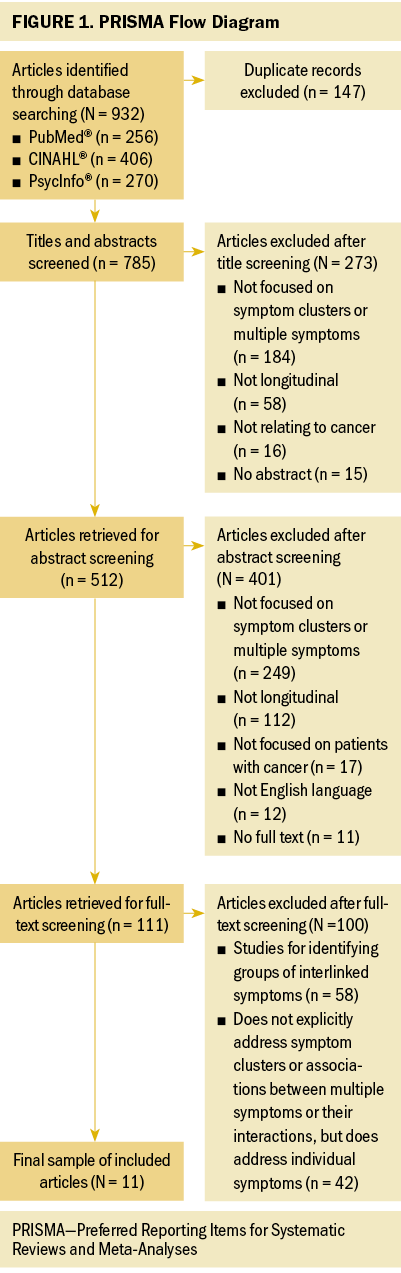
The initial search identified 932 articles (see Figure 1). After removing 147 duplicates through reference management software, 785 potential articles were identified for inclusion. After reviewing the titles, 273 were excluded and 512 were identified for review. After reviewing the abstracts, 401 were excluded and 111 were left for the full-text review. After being assessed for eligibility and methodologic appraisal, 100 articles were excluded. Finally, 11 articles satisfied the criteria for full review and inclusion in this integrative review.
Study Characteristics
Study characteristics are presented in Table 3. All 11 studies were published after 2010, and 7 were published in 2015 or after. The total scores of study quality were calculated using the QualSyst quality rating scale. The authors used relatively conservative cut points (more than 75%) and relatively liberal (more than 55%) quality criteria in line with recommendations from Kmet et al. (2004). The average quality rating was 94% (range = 80%–100%). All articles met the minimum standard for quality.
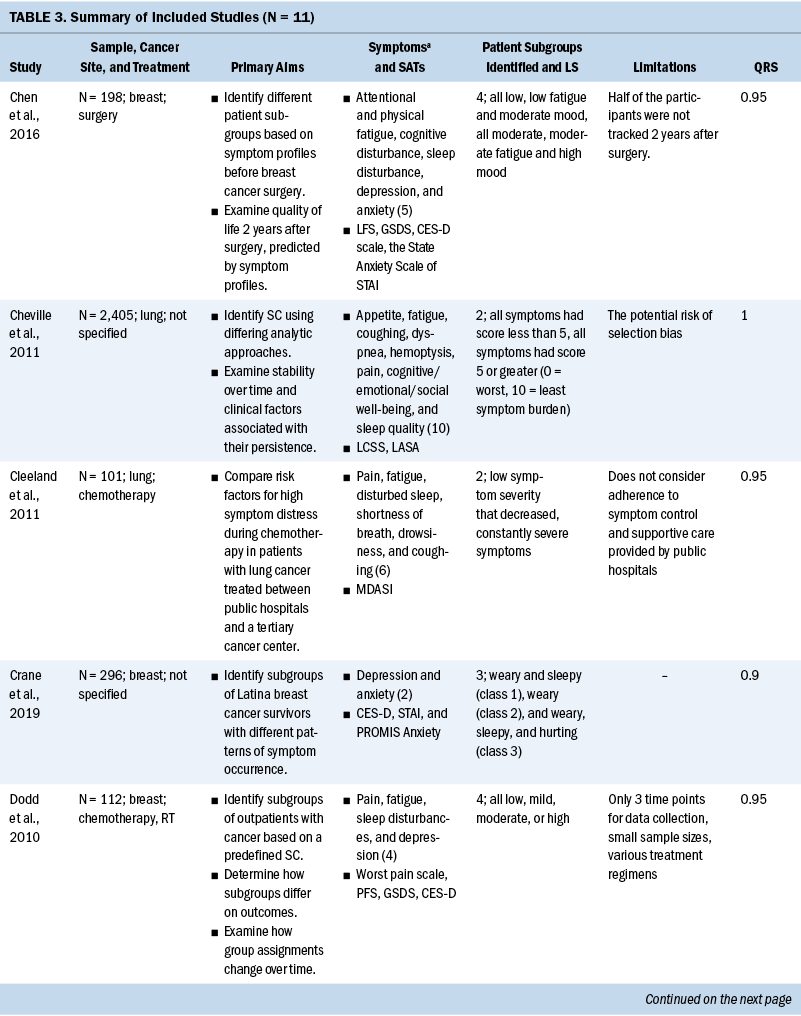
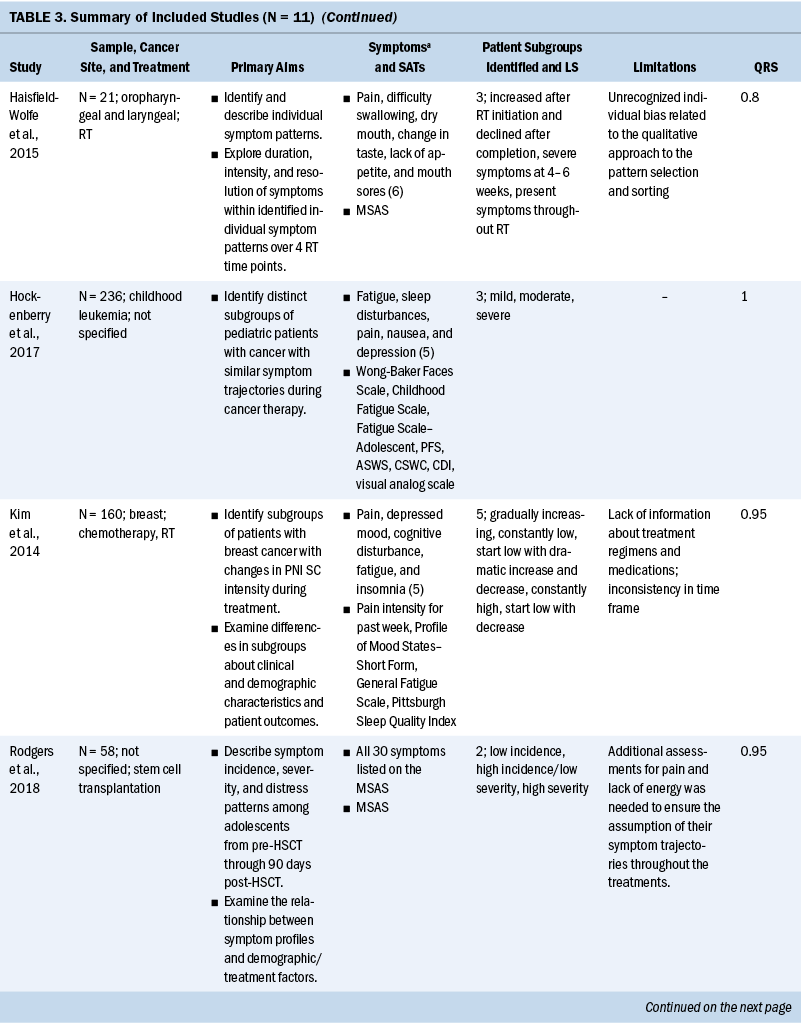
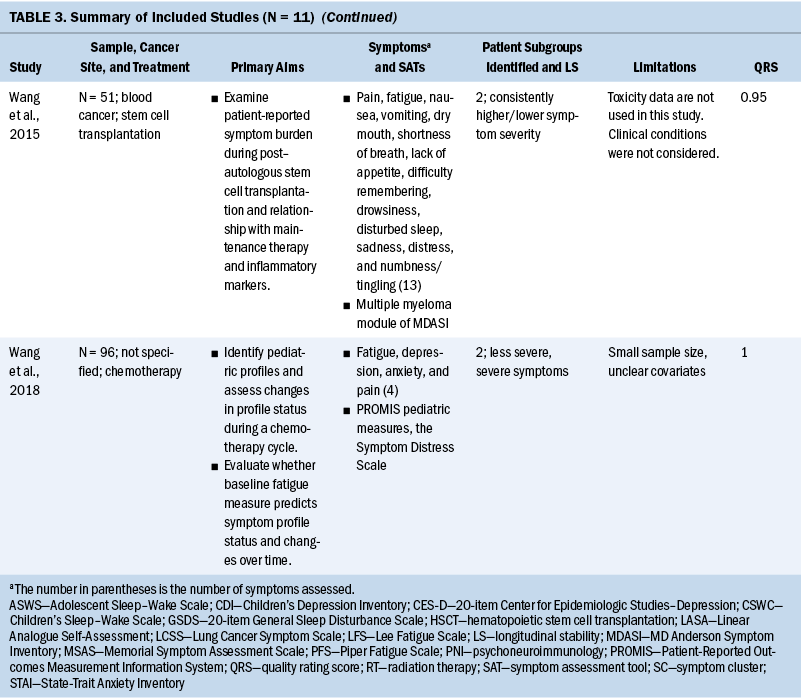
Ten of the 11 studies had a sample size range of 21–296, and 1 study (Cheville et al., 2011) had 2,405. Three of the 11 studies focused exclusively on women (Chen et al., 2016; Crane et al., 2019; Dodd et al., 2010), and the percentage range of female participants across the remaining 8 studies was 14% to 82.6%. One study specifically recruited Latina breast cancer survivors (Crane et al., 2019), but the racial and ethnic background of the remaining 10 studies was exclusively White. Primary cancer sites varied across the studies, with the most frequently studied primary site being breast (36%) (Chen et al., 2016; Crane et al., 2019; Dodd et al., 2010; Kim et al., 2014), followed by lung (18%) (Cheville et al., 2011; Cleeland et al., 2011), head and neck (9%) (Haisfield-Wolfe et al., 2015), childhood leukemia (9%) (Hockenberry et al., 2017), and blood cancer (9%) (Wang et al., 2015). Two of the 11 studies (18%) (Rodgers et al., 2018; Wang et al., 2018) did not limit inclusion to any specific cancer diagnosis.
Aim 1: Subgroups Based on Symptom Experiences and Patterns of Symptom Changes Over Time
Symptom assessments and approaches to data collection: There was little overlap in the specific symptom assessment tools used to collect symptom data. The most commonly used symptom assessment tools in the studies were the Memorial Symptom Assessment Scale (18%) (Haisfield-Wolfe et al., 2015; Rodgers et al., 2018) and the MD Anderson Symptom Inventory (18%) (Cleeland et al., 2011; Wang et al., 2015). One study measured three symptom dimensions, including incidence, severity, and distress, and the majority (91%) of studies only measured symptom severity.
Common SCs: Numerous symptoms were assessed across the studies, ranging from 2 to 30 symptoms. Pain was a focus in all studies (91%) but one (Crane et al., 2019). The next most commonly studied symptoms were sleep disturbance (73%) (Chen et al., 2016; Crane et al., 2019; Dodd et al., 2010; Hockenberry et al., 2017; Kim et al., 2014; Rodgers et al., 2018; Wang et al., 2015, 2018) and fatigue (73%) (Chen et al., 2016; Cheville et al., 2011; Cleeland et al., 2011; Dodd et al., 2010; Hockenberry et al., 2017; Kim et al., 2014; Wang et al., 2015, 2018), followed by depression (55%) (Chen et al., 2016; Crane et al., 2019; Dodd et al., 2010; Hockenberry et al., 2017; Kim et al., 2014; Wang et al., 2018).
The most commonly used SCs were the psychoneuroimmunology (PNI) cluster (typically defined as depressive symptoms, anxiety, cognitive dysfunction, fatigue, sleep disturbance, and pain), assessed in 7 of the 11 studies (Cheville et al., 2011; Cleeland et al., 2011; Dodd et al., 2010; Hockenberry et al., 2017; Kim et al., 2014; Wang et al., 2015, 2018). Symptoms of PNI mixed with the gastrointestinal (GI) cluster (typically defined as lack of appetite, nausea, taste changes, and bloating) were co-assessed in 1 of the 11 studies (Wang et al., 2015). An SC of pain, fatigue, sleep disturbance, and mood change was assessed in 4 of the 11 studies (Cheville et al., 2011; Dodd et al., 2010; Hockenberry et al., 2017; Kim et al., 2014). It is important to report that there are differences across the studies in the specific symptoms contained in the PNI and GI SC. Not all studies included all six PNI symptoms or all four GI symptoms.
Longitudinal stability: Ten of the 11 studies observed that the symptom experience of patients with cancer changed over time across their treatment trajectories (Cheville et al., 2011; Cleeland et al., 2011; Crane et al., 2019; Dodd et al., 2010; Haisfield-Wolfe et al., 2015; Hockenberry et al., 2017; Kim et al., 2014; Rodgers et al., 2018; Wang et al., 2015, 2018), and 1 study reported that symptom severity for each group of patients with multiple myeloma was stable during six months post–autologous stem cell transplantation (Wang et al., 2015). The remaining study collected symptom data at 12 time points, but overall mean scores of symptom measures were used to classify patients rather than 12 individual symptom assessments; therefore, these data have a limited ability to identify patterns of symptom changes over time (Chen et al., 2016).
Based on the 11 articles reviewed for this analysis, SCs varied based on the cancer diagnoses of the sample. Cluster analysis in lung cancer survivors revealed that rate changes in the transition across groups of SCs were most distinct in the time period between years one and two after diagnosis. Cluster analysis in women with breast cancer showed that subgroup assignments started from mild at the initiation of treatment and changed to moderate to severe symptoms after treatment (Dodd et al., 2010). Symptom patterns for adolescents experiencing hematopoietic stem cell recovery showed that the linear effects of mild and severe symptom trajectories showed little symptom change over time. Mild symptoms remained stable, and more severe symptom patterns peaked at 30 days after hematopoietic stem cell recovery and then gradually decreased. In children undergoing chemotherapy, cluster analysis showed the incidence of intense symptom profiles were stable from the beginning to the middle of the chemotherapy cycle but significantly declined after hematopoietic recovery following chemotherapy (Wang et al., 2018).
Patterns of symptom dimensions: Four studies reported that a majority of patients with cancer experience mild to moderate symptoms over time, and some patients experience severe symptoms over time (Cleeland et al., 2011; Crane et al., 2019; Hockenberry et al., 2017; Wang et al., 2015). Seventy percent of patients with advanced lung cancer reported mild symptom severity levels that declined throughout treatment, and 30% of patients had consistently severe symptoms throughout treatment (Cleeland et al., 2011). Hockenberry et al. (2017) identified three latent classes of symptom profiles with children undergoing treatment for leukemia. Looking at the symptoms of pain, fatigue, sleep disturbance, nausea, and depression in children receiving treatment for leukemia, 36% experienced mild symptoms, 52.2% experienced moderate symptoms, and 11.1% experienced severe symptoms (Hockenberry et al., 2017). Trajectory analysis in patients with multiple myeloma found that nearly 35% of these patients fit into a continuously severe symptoms group and 65% of patients consistently reported lower symptom severity for fatigue, pain, numbness/tingling, bone aches, and muscle weakness (Wang et al., 2015). Group-based growth mixture modeling in Latina breast cancer survivors identified that the majority of women experienced a low/moderate stable pattern of depression and a low stable pattern of anxiety (Crane et al., 2019).
One article reported that a majority of patients with cancer experienced severe symptoms over time (Rodgers et al., 2018). Symptom trajectory analysis of young patients during stem cell transplantation recovery revealed severe symptom trajectories for 39.5% of adolescents with initially mild symptom levels and 60.5% for patients experiencing higher symptom severity (Rodgers et al., 2018).
Aim 2: Methodologies for Identifying Subgroups of Patients With Cancer Based on Symptom Experience
Study design and approaches to data collection: The average number of individual symptom assessment points was 6, with a range of 3–15 assessment points. Eight of the 11 studies used three to five time points to measure symptom changes over time (Cheville et al., 2011; Crane et al., 2019; Dodd et al., 2010; Haisfield-Wolfe et al., 2015; Hockenberry et al., 2017; Kim et al., 2014; Rodgers et al., 2018; Wang et al., 2018). The timespan between assessments was diverse and varied from weekly, monthly, bimonthly, or every four months after the start of treatment. All studies collected symptom data using prospective surveys, and no studies used routinely collected health data.
Methods for subgroup identification: For the articles included in this review, the authors categorized each analysis technique used to group patients based on the following four subgroups: latent variable models, dimension reduction/clustering, basic statistical model, or ad hoc/qualitative. Some studies used two or more methods for data analysis and are represented in more than one subgroup.
Latent variable modeling was used for eight studies (Chen et al., 2016; Cheville et al., 2011; Cleeland et al., 2011; Crane et al., 2019; Hockenberry et al., 2017; Rodgers et al., 2018; Wang et al., 2015, 2018). Types of latent variable models include latent class analysis used with categorical variables, latent profile analysis used with continuous variables, latent transition analysis used to evaluate shifts in latent profile status over time, and a latent trait analysis used to derive information about continuous latent variables from the observed values of categorical variables (Hagenaars & McCutcheon, 2002; Magidson & Vermunt, 2002; Muthén & Muthén, 2004; Wang et al., 2018). Among these, two studies assessed only symptom group membership using a latent profile, class/trait analysis (Chen et al., 2016; Wang et al., 2018). In addition, seven studies used latent variable models to investigate not only group membership, but also trends as follows: latent class growth analysis, latent transition analysis, group-based trajectory modeling, and group-based growth mixture modeling (Cheville et al., 2011; Cleeland et al., 2011; Crane et al., 2019; Hockenberry et al., 2017; Rodgers et al., 2018; Wang et al., 2015, 2018). One study used multiple exploratory analyses (cluster and factor analysis) to validate an SC and then confirmatory models (latent trait analysis) to examine the consistency of an identified SC in a cohort of lung cancer survivors (Cheville et al., 2011).
Dimension reduction and clustering was used by three studies. Two studies used nonparametric clustering: hierarchical cluster analysis (Dodd et al., 2010) and exploratory factor analysis and principal component analysis (Cheville et al., 2011). The final study used a least-squares clustering method called Ward’s minimum variance (Kim et al., 2014). One study employed basic statistical modeling, one-way analysis of variance, and analysis of covariance to analyze differences between group means in symptom severity for each symptom assessed (Chen et al., 2016). One study used an ad hoc/qualitative method: visual graphical analysis (Haisfield-Wolfe et al., 2015), an exploratory approach in which each variable is graphed and examined to understand trajectories over time (Brown et al., 2007). The summary score (total sum/total possible sum) is more than 0.8 in 10 of the 11 studies, indicating the chosen studies were of acceptable quality for inclusion in this review.
Discussion
As part of this integrative review, the authors found that sample characteristics, symptom assessments, end points, methodologies, and outcomes varied considerably among the included studies. Although patient symptoms have various dimensions and change over time, the patient subgroups and symptom trajectories varied widely because of differences in type of cancer, treatment, and symptoms assessed, and diverse methodologies were used for SC analysis based on the different types of data, with each analysis method having unique benefits and limitations.
Aim 1: Patterns of Symptom Changes Over Time Within Patient Groups
There was variability in the number of groups identified with various symptom changes. However, the majority of studies identified two or three groups of patients with similar symptom experiences. Two (Cheville et al., 2011; Cleeland et al., 2011; Rodgers et al., 2018; Wang et al., 2015, 2018), three (Crane et al., 2019; Haisfield-Wolfe et al., 2015; Hockenberry et al., 2017), four (Chen et al., 2016; Dodd et al., 2010), or five (Kim et al., 2014) distinct patient groups were identified from symptom reports. This result can inform the number of groups that can be used in future studies involving clustering patients with different symptom experiences.
The authors found some inconsistencies in how groups of patients with cancer with similar symptom experiences are operationalized, and many studies did not include a rationale for selecting symptoms and how selected symptoms related to each other. This result suggests the need for methodologic evaluation by professional organizations or groups of measurement experts when defining SC and testing mechanisms for SC in future studies.
Aim 2: Methodologies for Identifying Subgroups Based on Symptom Experiences
Currently, there are no specified or preferred analytic approaches for identifying groups of individuals who share similar symptom experiences because this is a developing concept. The 11 studies used diverse methodologies, but a majority (8 of 11) of the studies used a latent variable model (Chen et al., 2016; Cheville et al., 2011; Cleeland et al., 2011; Crane et al., 2019; Hockenberry et al., 2017; Rodgers et al., 2018; Wang et al., 2015, 2018). Each analytic method has benefits and limitations to capture patterns of symptom changes. The benefits of latent variable models to create subgroups of symptom experience in patients is that more complex latent variable models can be applied and tested that better reflect the complex realities of data collected in symptom science research (Cai, 2012).
Hockenberry et al. (2017) used a latent class growth analysis to categorize children with leukemia into groups with similar patterns of change in symptoms, called latent classes; thus, researchers can identify the patients’ symptom trajectories over time. Cleeland et al. (2011) applied group-based trajectory modeling to sort individuals into groups with regard to symptom experiences based on either high or low levels and trajectories over time. Group-based trajectory modeling models within-group averages but not individual deviation from the group means. In contrast, latent-growth mixture modeling estimates individual variability within groups, informing how closely individuals within a group are similar to the mean.
Kim et al. (2014) applied Ward’s minimum variance method, which is based on the sum of squared errors, to identify groups of patients with breast cancer with different patterns of psychoneurologic symptom changes over the course of treatments. Haisfield-Wolfe et al. (2015) used visual graphical analysis to describe individual symptom patterns in symptom severity in a pilot study of outpatients with oropharyngeal and laryngeal cancer during radiation therapy. The qualitative method of pattern selection and classifying is a limitation related to unknown individual bias, reproducibility, flexibility, and acceptance for future research, particularly with large data sets.
Although all 11 studies used a longitudinal design, the clustering analysis was conducted separately at each time point; therefore, it is not possible to generalize how symptom experiences fluctuate across treatment trajectories. Future research is needed to demonstrate the optimal approach for symptom assessment over time.
All studies used survey data to assess symptoms, and no studies used routinely collected health data. This approach to symptom research introduces bias favoring the experience of people willing and able to participate in prospective survey research. For example, the National Cancer Patient Experience Survey data reported that older adult female patients with chronic conditions were significantly less likely to participate in the research (McGrath-Lone et al., 2015). In addition, a limited number of symptoms are included in assessment instruments for patients with cancer. Symptom surveys used in cancer research range from 2 to 41 symptoms of interest (Cherwin, 2012). Depending on the number of symptoms included, the overall impact on the patient may not be adequately represented in the symptom science literature (Cherwin, 2012). Currently, a large amount of health data is routinely collected and saved in healthcare data repositories. These data sources provide an alternate source of symptom data free of the sampling bias of survey research, a unique chance to discover patient profiles that cannot be captured from surveys alone, and opportunities to use more data points with large-scale, rich information from multiple sources, and time-series records.
Identifying groups of interlinked symptoms using an empirical method, which is most commonly used, should be applied along with identifying subgroups of patients with cancer because this would offer established co-occurring symptoms and foundations that would be valuable for researchers and clinicians. Relevant variables that the authors did not examine (e.g., medications, immunotherapy, chemotherapy regimens, other patient diagnoses) can be collected for other studies, depending on the research focus.
Longitudinal studies that group patients with cancer with similar symptom experiences on a preselected set of symptoms are new to SC science and perhaps not well understood. Similarly, a previous review of the literature by Dong et al. (2014) revealed there is no consensus about methodologies for grouping patients, and studies or efforts for evaluating methods are currently hard to find. Clinicians and researchers should consider a more comprehensive examination of time frame, symptom measurement tools that include key symptoms for patients with cancer, routinely gathered clinical data collection, and innovative data analysis methods with the context of cancer.
Limitations
Overall limitations of the studies in this review include small sample sizes; various types of treatments, which could affect symptom profiles; potential risk of selection bias; or unclear covariates. The limitations on making conclusions about the studies is not because of the quality of the studies; it is because of heterogeneity and variability in approach to measuring symptoms and subgrouping symptom experiences in patients over time. The results of this study emphasize the importance of development of extensive and more accurate methodologies in the future to identify patient phenotypes of symptom experiences. Given the diversity of cancer types, treatments, and symptom assessments of studies, true synthesis may not be achievable. Future research is needed to focus SC analyses in individual types of cancer rather than many combined cancer diagnoses, which would provide evidence for disease-specific symptom profiles and more direct clinical application for symptom management interventions. In addition, the authors’ search was limited to full-text articles published in English, thus excluding non-English articles and conference abstracts.
Implications for Nursing
Clinicians can be informed which patients have a high risk of severe symptom burden over time compared to other groups of patients and can plan treatments and symptom management based on this knowledge. Researchers who want to group patients based on different symptom experiences across treatment trajectories can consider diverse methods based on this methodologic summary of previous studies. The authors’ goal was not to identify SCs and how they related to different outcomes; rather, the goal of this review was to summarize and critique the methodology for grouping people with similar symptom experiences over time.
Conclusion
This article examines the literature focusing on identifying subgroups of symptom experiences in patients with cancer based on similar patterns of longitudinal symptom experiences, describing subgroup membership changes and the extent to which different methodologies have been used in this research. The authors identified the benefits and limitations of methodologies for subgrouping symptom experiences in patients with cancer and what researchers should consider when they choose a method. There is significant variability in the literature, including variation in the sample (i.e., age, sex), cancer types and treatments, symptom assessment tools, and analytic methods. Of note, there is no consensus about common standards or evaluations for how to categorize and group patients with similar symptom experiences. Innovative methods to cluster patients assist clinicians and researchers in identifying meaningful groups sharing similar symptom experiences during cancer treatment.
The authors gratefully acknowledge Jennifer DeBerg, MLS, for her assistance with the systematic search and Priska Anugom, RN, PHN, BSN, for data extraction.
About the Authors
Sena Chae, PhD, RN, is an associate faculty member in the College of Nursing, Catherine Cherwin, PhD, RN, is an assistant professor in the College of Nursing, W. Nick Street, PhD, is a professor in the Tippie College of Business, Sue Moorhead, RN, PhD, FAAN, is an associate professor emeritus in the College of Nursing, Grant Brown, PhD, is an assistant professor in the College of Public Health, and Stephanie Gilbertson-White, PhD, APRN-BC, is an associate professor in the College of Nursing, all at the University of Iowa in Iowa City. Chae, Street, and Gilbertson-White contributed to the conceptualization and design. Chae completed the data collection. Brown provided statistical support. Chae, Cherwin, Brown, and Gilbertson-White provided the analysis. All authors contributed to the manuscript preparation. Chae can be reached at sena-chae@uiowa.edu, with copy to ONFEditor@ons.org. (Submitted June 2021. Accepted November 24, 2021.)
References
Ameringer, S., Elswick, R.K., Jr., Shockey, D.P., & Dillon, R. (2013). A pilot exploration of symptom trajectories in adolescents with cancer during chemotherapy. Cancer Nursing, 36(1), 60–71. https://doi.org/10.1097/NCC.0b013e318250da1a
Bender, C.M., Engberg, S.J., Donovan, H.S., Cohen, S.M., Houze, M.P., Rosenzweig, M.Q., . . . Sereika, S.M. (2008). Symptom clusters in adults with chronic health problems and cancer as a comorbidity. Oncology Nursing Forum, 35(1), E1–E11. https://doi.org/10.1188/08.ONF.E1-E11
Brant, J.M., Beck, S.L., Dudley, W.N., Cobb, P., Pepper, G., & Miaskowski, C. (2011). Symptom trajectories during chemotherapy in outpatients with lung cancer colorectal cancer, or lymphoma. European Journal of Oncology Nursing, 15(5), 470–477. https://doi.org/10.1016/j.ejon.2010.12.002
Brown, C.G., McGuire, D.B., Beck, S.L., Peterson, D.E., & Mooney, K.H. (2007). Visual graphical analysis: A technique to investigate symptom trajectories over time. Nursing Research, 56(3), 195–201. https://doi.org/10.1097/01.NNR.0000270029.82736.5a
Cai, L. (2012). Latent variable modeling. Shanghai Archives of Psychiatry, 24(2), 118–120. https://doi.org/10.3969/j.issn.1002-0829.2012.02.010
Chen, M.L., Liu, L.N., Miaskowski, C., Chen, S.C., Lin, Y.C., & Wang, J.S. (2016). Presurgical symptom profiles predict quality of life 2 years after surgery in women with breast cancer. Supportive Care in Cancer, 24(1), 243–251. https://doi.org/10.1007/s00520-015-2784-8
Cherwin, C.H. (2012). Gastrointestinal symptom representation in cancer symptom clusters: A synthesis of the literature. Oncology Nursing Forum, 39(2), 157–165. https://doi.org/10.1188/12.ONF.157-165
Cheville, A.L., Novotny, P.J., Sloan, J.A., Basford, J.R., Wampfler, J.A., Garces, Y.I., . . . Yang, P. (2011). Fatigue, dyspnea, and cough comprise a persistent symptom cluster up to five years after diagnosis with lung cancer. Journal of Pain and Symptom Management, 42(2), 202–212. https://doi.org/10.1016/j.jpainsymman.2010.10.257
Cleeland, C.S., Mendoza, T.R., Wang, X.S., Woodruff, J.F., Palos, G.R., Richman, S.P., . . . Lu, C. (2011). Levels of symptom burden during chemotherapy for advanced lung cancer: Differences between public hospitals and a tertiary cancer center. Journal of Clinical Oncology, 29(21), 2859–2865. https://doi.org/10.1200/jco.2010.33.4425
Crane, T.E., Badger, T.A., Sikorskii, A., Segrin, C., Hsu, C.H., & Rosenfeld, A.G. (2019). Trajectories of depression and anxiety in Latina breast cancer survivors. Oncology Nursing Forum, 46(2), 217–227. https://doi.org/10.1188/19.ONF.217-227
Dodd, M., Miaskowski, C., & Paul, S.M. (2001). Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum, 28(3), 465–470.
Dodd, M.J., Cho, M.H., Cooper, B.A., & Miaskowski, C. (2010). The effect of symptom clusters on functional status and quality of life in women with breast cancer. European Journal of Oncology Nursing, 14(2), 101–110. https://doi.org/10.1016/j.ejon.2009.09.005
Dodd, M.J., Miaskowski, C., & Lee, K.A. (2004). Occurrence of symptom clusters. JNCI Monographs, 2004(32), 76–78. https://doi.org/10.1093/jncimonographs/lgh008
Dong, S.T., Butow, P.N., Costa, D.S.J., Lovell, M.R., & Agar, M. (2014). Symptom clusters in patients with advanced cancer: A systematic review of observational studies. Journal of Pain and Symptom Management, 48(3), 411–450. https://doi.org/10.1016/j.jpainsymman.2013.10.027
Doong, S.H., Dhruva, A., Dunn, L.B., West, C., Paul, S.M., Cooper, B.A., . . . Miaskowski, C. (2015). Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biological Research for Nursing, 17(3), 237–247. https://doi.org/10.1177/1099800414550394
Fan, G., Filipczak, L., & Chow, E. (2007). Symptom clusters in cancer patients: A review of the literature. Current Oncology, 14(5), 173–179. https://doi.org/10.3747/co.2007.145
Fox, S.W., & Lyon, D.E. (2006). Symptom clusters and quality of life in survivors of lung cancer. Oncology Nursing Forum, 33(5), 931–936. https://doi.org/10.1188/06.ONF.931-936
Gilbertson-White, S., Aouizerat, B.E., Jahan, T., & Miaskowski, C. (2011). A review of the literature on multiple symptoms, their predictors, and associated outcomes in patients with advanced cancer. Palliative and Supportive Care, 9(1), 81–102. https://doi.org/10.1017/s147895151000057x
Gold, M., Dunn, L.B., Phoenix, B., Paul, S.M., Hamolsky, D., Levine, J.D., & Miaskowski, C. (2016). Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. European Journal of Oncology Nursing, 20, 97–105. https://doi.org/10.1016/j.ejon.2015.06.003
Hagenaars, J.A., & McCutcheon, A.L. (2002). Applied latent class analysis. Cambridge University Press.
Haisfield-Wolfe, M.E., Brown, C., Richardson, M., & Webster, K. (2015). Variations in symptom severity patterns among oropharyngeal and laryngeal cancer outpatients during radiation treatment: A pilot study. Cancer Nursing, 38(4), 279–287. https://doi.org/10.1097/ncc.0000000000000183
Henoch, I., & Lövgren, M. (2014). The influence of symptom clusters and the most distressing concerns regarding quality of life among patients with inoperable lung cancer. European Journal of Oncology Nursing, 18(3), 236–241. https://doi.org/10.1016/j.ejon.2013.12.001
Hockenberry, M.J., Hooke, M.C., Rodgers, C., Taylor, O., Koerner, K.M., Mitby, P., . . . Pan, W. (2017). Symptom trajectories in children receiving treatment for leukemia: A latent class growth analysis with multitrajectory modeling. Journal of Pain and Symptom Management, 54(1), 1–8. https://doi.org/10.1016/j.jpainsymman.2017.03.002
Hwang, K.H., Cho, O.H., & Yoo, Y.S. (2016). Symptom clusters of ovarian cancer patients undergoing chemotherapy, and their emotional status and quality of life. European Journal of Oncology Nursing, 21, 215–222. https://doi.org/10.1016/j.ejon.2015.10.007
Hwang, S.S., Chang, V.T., Fairclough, D.L., Cogswell, J., & Kasimis, B. (2003). Longitudinal quality of life in advanced cancer patients: Pilot study results from a VA medical cancer center. Journal of Pain and Symptom Management, 25(3), 225–235. https://doi.org/10.1016/S0885-3924(02)00641-3
Illi, J., Miaskowski, C., Cooper, B., Levine, J.D., Dunn, L., West, C., . . . Baggott, C. (2012). Association between pro-and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine, 58(3), 437–447. https://doi.org/10.1016/j.cyto.2012.02.015
Kim, H.J., Barsevick, A.M., Beck, S.L., & Dudley, W. (2012). Clinical subgroups of a psychoneurologic symptom cluster in women receiving treatment for breast cancer: A secondary analysis. Oncology Nursing Forum, 39(1), E20–E30. https://doi.org/10.1188/12.ONF.E20-E30
Kim, H.J., McDermott, P.A., & Barsevick, A.M. (2014). Comparison of groups with different patterns of symptom cluster intensity across the breast cancer treatment trajectory. Cancer Nursing, 37(2), 88–96. https://doi.org/10.1097/NCC.0b013e31828293e0
Kmet, L.M., Lee, R.C., & Cook, L.S. (2004). Standard quality assessment criteria for evaluating primary research papers from a variety of fields. Alberta Heritage Foundation for Medical Research, Health Technology Assesment Unit. https://www.ihe.ca/download/standard_quality_assessment_criteria_for_ev…
Kroenke, K., Johns, S.A., Theobald, D., Wu, J., & Tu, W. (2013). Somatic symptoms in cancer patients trajectory over 12 months and impact on functional status and disability. Supportive Care in Cancer, 21(3), 765–773. https://doi.org/10.1007/s00520-012-1578-5
Lee, L., Packer, T.L., Tang, S.H., & Girdler, S. (2008). Self-management education programs for age-related macular degeneration: A systematic review. Australasian Journal on Ageing, 27(4), 170-176. https://doi.org/10.1111/j.1741-6612.2008.00298.x
Lin, S., Chen, Y., Yang, L., & Zhou, J. (2013). Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. Journal of Clinical Nursing, 22(9–10), 1281–1290. https://doi.org/10.1111/jocn.12228
Magidson, J., & Vermunt, J. (2002). Latent class models for clustering: A comparison with K-means. Canadian Journal of Marketing Research, 20, 36–43.
McGrath-Lone, L., Day, S., Schoenborn, C., & Ward, H. (2015). Exploring research participation among cancer patients: Analysis of a national survey and an in-depth interview study. BMC Cancer, 15, 618. https://doi.org/10.1186/s12885-015-1628-8
Miaskowski, C., Aouizerat, B.E., Dodd, M., & Cooper, B. (2007). Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. JNCI Monographs, 2007(37), 39–46. https://doi.org/10.1093/jncimonographs/lgm003
Miaskowski, C., Barsevick, A., Berger, A., Casagrande, R., Grady, P.A., Jacobsen, P., . . . Marden, S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of the National Cancer Institute, 109(4), djw253. https://doi.org/10.1093/jnci/djw253
Miaskowski, C., Cooper, B.A., Paul, S.M., Dodd, M., Lee, K., Aouizerat, B.E., . . . Bank, A. (2006). Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncology Nursing Forum, 33(5), E79–E89. https://doi.org/10.1188/06.ONF.E79-E89
Muthén, L.K., & Muthén, B.O. (2004). Mplus user’s guide: Statistical analysis with latent variables. Muthén & Muthén.
National Institute of Nursing Research. (2019). Spotlight on symptom science and nursing research. U.S. Department of Health and Human Services, National Instutites of Health. https://www.ninr.nih.gov/researchandfunding/spotlights-on-nursing-resea…
Nho, J.H., Kim, S.R., & Nam, J.H. (2017). Symptom clustering and quality of life in patients with ovarian cancer undergoing chemotherapy. European Journal of Oncology Nursing, 30, 8–14.
Phligbua, W., Pongthavornkamol, K., Knobf, T.M., Junda, T., Viwatwongkasem, C., & Srimuninnimit, V. (2013). Symptom clusters and quality of life in women with breast cancer receiving adjuvant chemotherapy. Pacific Rim International Journal of Nursing Research, 17(3), 249–267. https://he02.tci-thaijo.org/index.php/PRIJNR/article/view/7339
Rha, S.Y., & Lee, J. (2017). Symptom clusters during palliative chemotherapy and their influence on functioning and quality of life. Supportive Care in Cancer, 25(5), 1519–1527. https://doi.org/10.1007/s00520-016-3545-z
Rodgers, C., Highberger, M., Powers, K., Voigt, K., & Douglas, C. (2018). Symptom trajectories of adolescents during hematopoietic stem cell recovery. Cancer Nursing, 42(6), 468–474. https://doi.org/10.1097/ncc.0000000000000643
Ryu, E., Kim, K., Cho, M.S., Kwon, I.G., Kim, H.S., & Fu, M.R. (2010). Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nursing, 33(1), 3–10. https://doi.org/10.1097/NCC.0b013e3181b4367e
Thavarajah, N., Chen, E., Zeng, L., Bedard, G., Di Giovanni, J., Lemke, M., . . . Chow, E. (2012). Symptom clusters in patients with metastatic cancer: A literature review. Expert Review of Pharmacoeconomics and Outcomes Research, 12(5), 597–604. https://doi.org/10.1586/erp.12.41
Wang, J., Jacobs, S., Dewalt, D.A., Stern, E., Gross, H., & Hinds, P.S. (2018). A longitudinal study of PROMIS pediatric symptom clusters in children undergoing chemotherapy. Journal of Pain and Symptom Management, 55(2), 359–367. https://doi.org/10.1016/j.jpainsymman.2017.08.021
Wang, X.S., Shi, Q., Williams, L.A., Shah, N.D., Mendoza, T.R., Cohen, E.N., . . . Orlowski, R.Z. (2015). Longitudinal analysis of patient-reported symptoms post-autologous stem cell transplant and their relationship to inflammation in patients with multiple myeloma. Leukemia and Lymphoma, 56(5), 1335–1341. https://doi.org/10.3109/10428194.2014.956313
Whittemore, R., & Knafl, K. (2005). The integrative review: Updated methodology. Journal of Advanced Nursing, 52(5), 546–553. https://doi.org/10.1111/j.1365-2648.2005.03621.x




