Microbial Growth on the Nails of Direct Patient Care Nurses Wearing Nail Polish
Objectives: To determine whether nurses wearing nail polish pose a greater infection risk to patients than nurses who are not wearing nail polish.
Sample & Setting: 89 direct patient care oncology nurses at a large midwestern National Cancer Institute–designated comprehensive cancer center.
Methods & Variables: The investigators assigned participants’ three middle fingers of their dominant hand to three groups: no nail polish, one-day-old polish, and four-day-old polish at the time of culture. Standard nail polish was applied using a consistent technique. Participants were required to work a shift immediately prior to nail cultures and practice routine hospital hand hygiene. Bacterial cultures were obtained from the nonpolished nail and the polished nails when the polish was one day old and four days old.
Results: Comparison of colony-forming units revealed that one-day-old polish exhibited fewer gram-positive microorganisms than the unpolished nail (p = 0.04). The four-day-old polish showed significantly more microorganisms than the one-day-old polish (p = 0.03). The same trend was demonstrated for gram-negative microorganisms, but the difference was not statistically significant (p = 0.3 and p = 0.17, respectively).
Implications for Nursing: The results should be interpreted and applied to expert nursing practice in the care of vulnerable patient populations. Each institution and practitioner should make their own decisions and interpretation of evidence into practice.
Jump to a section
The link between caregivers’ hands and transmission of pathogenic organisms has been discussed since hand hygiene was first implemented by Semmelweis to reduce the incidence of puerperal fever (Nuland, 2004). It is widely accepted that caregivers’ hands are a vehicle for transmitting health care–acquired infection (HAI) pathogens (Siegel et al., 2019). Improved efficacy of hand hygiene among direct patient care nurses is of vital concern in the oncology setting because immunocompromised patients are at high risk for morbidity and mortality associated with HAIs (Abou Dagher et al., 2017). Although the process of hand hygiene has been studied extensively, less is known about the effect that wearing nail polish has on the growth of potentially pathogenic microbes on the hands of direct patient care nurses outside of the operative setting.
Nursing dress code policy may vary among institutions, but it lacks a basis in research evidence regarding the use of nail polish by direct patient care staff (Cimon & Featherstone, 2017). A review of the historic literature revealed that evidence supports dress codes banning artificial nails in the operative setting and for individuals providing direct patient care (Gordin et al., 2007; McNeil et al., 2001; Pottinger et al., 1989; Wynd et al., 1994).
This study hypothesized that wearing nail polish likely increases microbes retained at the junction of the polish and nail over time, despite routine healthcare hand hygiene. The investigators sought to generate evidence-based recommendations for improving nursing infection prevention practice and dress code policy. The conceptual framework for this study is based on the five-step sequence of transmission of microbes via healthcare providers’ hands according to the evidence-based model for hand transmission during patient care, as shown in Figure 1. 
The transmission of pathogenic organisms to susceptible hosts can occur in multiple ways. One example is through the mode of indirect contact. Hands of healthcare providers may transmit pathogens after touching an infected or colonized body site on one patient or a contaminated inanimate object if hand hygiene is not performed before touching another patient (Siegel et al., 2019). Hand hygiene is critical to preventing the spread of potential pathogens and is considered one of the most important steps in preventing HAIs. According to the Healthcare Infection Control Practices Advisory Committee’s guideline for hand hygiene in healthcare settings (Boyce & Pittet, 2002), “the hands of healthcare workers may become persistently colonized with pathogenic flora (e.g., Staph aureus), gram negative bacilli, and yeast” (p. 2). These pathogenic organisms can cause HAIs, which are detrimental to patient care. HAIs can have many adverse effects to patients, including increased length of hospital stay, readmission to the hospital, and additional surgical procedures. The Centers for Medicare and Medicaid Services and Joint Commission audit healthcare facilities’ HAI rates and financially penalize facilities if their HAI rates exceed benchmarks (McClung et al., 2017). Therefore, reducing HAI rates in healthcare settings is not only critical to improving patient safety and patient satisfaction, but also to the financial stability of the institution. One way to help reduce HAI rates is to ensure that adequate hand hygiene is performed consistently by every healthcare provider.
Significance
Individuals with cancer are among the most susceptible to illness, morbidity, and mortality related to infections, including infections acquired from the hospital (Ulrich et al., 2017). Numerous professional oncology organizations and professional groups, such as the Oncology Nursing Society (Wilson et al., 2018), American Society of Clinical Oncology (Schiffer et al., 2013), and National Comprehensive Cancer Network (2018), have published detailed recommendations for prevention and control of infection in patients with cancer. These recommendations are of utmost importance in this vulnerable patient population because of immunodeficiency that is associated with various cancers and cancer treatments (Johnson, 2014).
Patients with cancer are at risk for infection from their disease and the nature of necessary treatments. For instance, tumor-related erosion of normal anatomic barriers or obstruction of the respiratory, biliary, and genitourinary tracts can contribute to an increased risk of infection (DeVita et al., 2015). Neutropenia is a major risk factor for infections in patients receiving treatment. Lack of granulocytes facilitates bacterial and fungal infections and blunts the inflammatory response, allowing infections to progress much faster compared to patients who are not immunocompromised (DeVita et al., 2015). This leaves patients at risk for numerous viral, bacterial, and fungal infections. Cancer-related infections cause significant complications in cancer care, including delayed treatment, longer hospitalizations, and higher mortality (Wang et al., 2015).
Because infection is so common in the cancer population and immediate attention is needed to treat infections, multidrug-resistant organisms have become a challenge (Wilson et al., 2018). Sepsis, a frequent sequela of bloodstream infections in immunocompromised patients, has a high fatality rate in patients with cancer (Gudiol et al., 2016). Such examples illustrate why infection control in the environment of care and among professional caregivers is a critical patient safety issue.
Literature Review
Evidence related to nail adornments and infection prevention primarily arose from the operative and perioperative setting. Initially, investigators found that wearing artificial nails increased the bacterial load of fingernails (McNeil et al., 2001; Pottinger et al., 1989). By extrapolation, and an abundance of caution, guidelines were developed recommending that all healthcare providers remove not only artificial nails, but also nail polish, to minimize the risk of infection to patients in the operative and perioperative settings (Pittet et al., 2009).
Arrowsmith and Taylor (2014) published a systematic review that concluded no evidence exists to support that nail polish plays a role in postsurgical infection; however, they limited the review to users of surgical hand scrub. Two main studies (Baumgardner et al., 1993; Wynd et al., 1994) were presented in that systematic review. Baumgardner et al. (1993) conducted a small (N = 26) nonrandomized study of microbial growth on the nails of surgical staff members using routine hand washing. The study did not find any significant differences between numbers of colony-forming units (CFUs) on unpolished versus polished nails.
Wynd et al. (1994) conducted a randomized controlled trial (RCT) of microbial growth on unpolished, polished, or chipped nails of 102 surgical nurses. An insignificant difference in CFUs was found between the groups following a surgical scrub. The study did not address nonsurgical staff using routine hand hygiene and was considered underpowered on review (Arrowsmith & Taylor, 2014). In addition to the previously mentioned trials, Lingaas and Fagernes (2009) conducted a larger (N = 465) nonrandomized cross-sectional study of whole-hand bacterial cultures among healthcare providers. Again, no relationship was found between microbial growth and the presence or condition of nail polish. The study was significant because of the large sample size, inclusion of nonsurgical and non-nursing staff, and regression analysis involving two study periods.
A study by Hewlett et al. (2018) was conducted (N = 88) to evaluate the bacterial burden of gel-polished nails, standard nail polish, and natural nails. The investigators demonstrated that the bacterial burden of nails became greater over time for all nail types, but hand hygiene may be more effective with natural nails (Hewlett et al., 2018). Although the literature appears to indicate a lack of impact of wearing nail polish on the potential transmission of microbial pathogens by nurses, the limited strength and generalizability of results suggests a need for a larger, rigorous, current RCT.
The purpose of the current RCT was to compare differences in type and amount of bacteria on natural nails compared to those that are polished. In addition, the study served to determine if the age or condition of the polish made a difference in the bacterial load on nails.
Methods
Sample and Setting
The sample included 89 direct patient care oncology nurses at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute in Columbus, Ohio. Participant inclusion criteria were as follows:
• On the day cultures were collected, the nurses must have worked a shift immediately prior to culture collection.
• The nurses must not have had a manicure or nail polish applied within the month before participation.
• The nurses’ nails could not be exposed to artificial sources of ultraviolet (UV) light for the duration of participation.
Exclusion criteria for the study included nurses without full-time direct patient care duties and self-identified nail biters.
Data for the power analysis were derived from a Cochrane Review, which focused on nail polish and related infection (Arrowsmith & Taylor, 2014). This review provided three estimates of the standard deviation of CFU measures taken from surgical nurses before scrubbing. The standard deviations reported were 12,006 for freshly polished nails, 2,016 for unpolished nails, and 453 for chipped/old-polish nails. These estimates of how much the data vary from the average apply to CFU measurement variability in this study. In microbiology, CFU is a unit used to estimate the number of viable bacteria or fungal cells in a sample. Analyses based on logarithmic transformation of number of CFUs are common because the number of bacteria in a sample can be very large, resulting in an asymmetric distribution of CFU measures with a long tail in the positive direction. The three estimates of standard deviation provided in the Cochrane Review were transformed to a natural logarithm scale for sample size estimates in this study (Arrowsmith & Taylor, 2014). The average of the three transformed estimates was 3.5. The assumption was made that the primary analysis would include tests of within-subject differences in number of CFUs between unpolished nails and those with one-day-old and four-day-old polish at the time of culture. For these comparisons, it was estimated that 87 participants would provide a probability of at least 0.93 of detecting control/treatment differences of at least 1.75 CFUs on the logarithm scale, which is half of the assumed standard deviation. CFUs are a proxy measure for possible patient outcomes of contamination and infection. Previous studies used number of CFUs as a measurement of bacterial load on an object (e.g., healthcare providers’ hands, stethoscopes, water for dialysis). Higher bacterial loads are essentially more contaminated and, therefore, are larger potential vectors of infection to patients (Coulliette & Arduino, 2013). In their benchmark study, Wynd et al. (1994) asserted that a three-log reduction (relative to log base 10) in CFUs is clinically significant.
Recruitment for study participation was conducted via a flyer sent out to all nursing staff by email after institutional approval was obtained from the institutional review board. One hundred nurses were recruited to account for attrition. Eligibility requirements for the study and consent process were described in the flyer. Interested nurses had one week to reply by email to a dedicated email address. For each study participant, the three middle fingers of the dominant hand were assigned by randomization table to no polish, one-day-old polish, and four-day-old polish at the time of culture.
Procedure
After consent, participants were listed alphabetically on a randomization table. GraphPad Software was used to produce a random assignment of nails for each polish group. Polish groups indicated which of the three middle nails on the dominant hand was assigned as the unpolished nail, one-day-old polish nail, and four-day-old polish nail. All participants had their nails trimmed to a quarter of an inch prior to study participation, consistent with recommendations from the Centers for Disease Control and Prevention (2002) for hand hygiene. Participants were blinded to which polished nail was assigned to which culture group.
During the study, participants were instructed to perform hand hygiene as outlined on the hand hygiene educational handout, which was reviewed at the time of consent. Hand hygiene included performing a hand rub for 30 seconds with an alcohol-based formulation at specific times, including prior to patient contact; prior to aseptic task; after body fluid exposure risk, patient contact, and contact with patient surroundings; and before and after donning gloves. Alternatively, hand washing with soap and water for 60 seconds was to be performed when hands were visibly soiled, with specific infection precautions. Participants were instructed that they may not perform any type of manicure on their nails, including clipping, filing, painting, soaking, or removing polish, even if the polish became chipped or cracked during the study. They were also instructed not to apply anything over their nails, such as bandages or tape. Participants were not to have a manicure or have any nail polish applied for one month prior to the study to avoid affecting the study results. Participants were to have no exposure to artificial UV light during the study, such as nail-curing devices or tanning beds. UV light may cause polish to be harder and more resistant to wear than polish unexposed to UV light (Manetti, 2012), which may affect the study results.
Twelve volunteers from the institution’s nursing staff were recruited to act as manicurists. The volunteer manicurists were trained in a standardized nail polish application technique to ensure consistency. Each attended a 30-minute training session during which they demonstrated polishing one nail on themselves and one nail on another person. Training dictated that the nails were clean and completely dry prior to polish application. Nails were measured prior to polishing to ensure length of a quarter of an inch, plus or minus an eighth of an inch. Participants with nails exceeding the required length were provided with nail clippers and a nail file and instructed to adjust the nail length to the required measurement. A single stroke of acetone-based polish remover was applied just prior to polish to ensure that nails were dry and free of oils or lotions. A single-layer coat of polish was applied to the nail in three equal, adjacent strokes. Thirty seconds of timed, fan-assisted drying occurred after each of the first two coats. After the third and final coat of polish, participants were required to stay in the presence of the manicurist for five minutes of timed, fan-assisted drying. If a nail became smudged during this process, manicurists were instructed to finish the process on all other nails and then completely remove the polish from the smudged nail and reapply using the standardized process. Manicurists were allowed to clean up any areas where the polish touched the cuticle or finger by lightly scratching with an emery board. The manicurists were blinded to which polish group each nail was assigned. The polish used on each participant was a single shade of pink from one retail manufacturer.
All participants were required to work at least one full shift in their usual department in direct patient care prior to being cultured. Volunteers who obtained swabbed samples for cultures from each participant were not permitted to be study participants. Each of the three sample-collection volunteers attended a 15-minute training session, during which they demonstrated a consistent sampling technique. Samples were collected by swabbing the nail and subungual areas of the three middle fingers on the dominant hand and were concentrated on any areas of chipped polish. The sample collection volunteers were blinded to which nails were in the one-day-old group and which nails were in the four-day-old group. They were instructed by the principal investigator of the study to collect samples on specific nails using designated individual swabs for each nail. Therefore, only the nails that were actually scheduled to be cultured on that day were sent for culture. The other swabs were “dummies” and were discarded. The sample collection volunteers were also asked to rate each participant’s current polish status each day according to the chipping parameters in Figure 2. Culture swabs were immediately refrigerated after sampling and were delivered daily, in bulk, to the microbiology laboratory and processed the same day. Laboratory personnel were also blinded to nail group assignment. 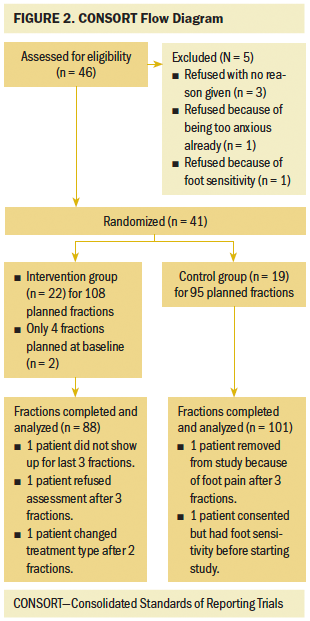
The Veriswab™ sampler with 4 ml neutralizing buffer was used for sampling. The Veriswab sampler includes a 16 x 100 mm polypropylene tube and screw-cap with an attached swab. The swab is Dacron-tipped, and the tube has a flat base that sits stably on a solid surface while a sample is being collected. Veriswab samplers are terminally sterilized by gamma radiation and remain sterile until the user removes the cap. The kit used comes prefilled with neutralizing buffer. Neutralizing buffer is used to collect a sample from a surface where hand sanitizer residue may be present.
All data analyses were performed using SAS, version 9.4, with CFUs as count data. Negative binomial regression and generalized Poisson regression were used to fit the regression models of CFUs. All modeling was done using the SAS procedure GLIMMIX. Significance was set at a priori of p ≤ 0.05. 
Results
The sample included 89 nurses who completed the study for a total sample size of 267 nails. Full demographics for the participants in the study are listed in Table 1. Outpatient nurses outnumbered inpatient nurses by more than three to one, and one male nurse participated in the study. All of the other demographics reflect the composition of the nursing staff as a whole. Eighty-nine nurses participated in the study, but two participants had to withdraw prior to the final-day cultures because of illness, leaving data from 265 nails for analysis, as reflected in the CONSORT diagram in Figure 3. 
In reference to whether polished nails carried a greater number of microorganisms, the mean number of CFUs for gram-positive organisms was 764, and the mean number of CFUs for gram-negative organisms was 60. Gram-positive microorganisms are more commonly found on the hands of healthcare providers (Pan et al., 2014). For gram-positive and gram-negative organisms, Table 2 provides the mean number of observed CFUs for each of the nail polish treatment levels. 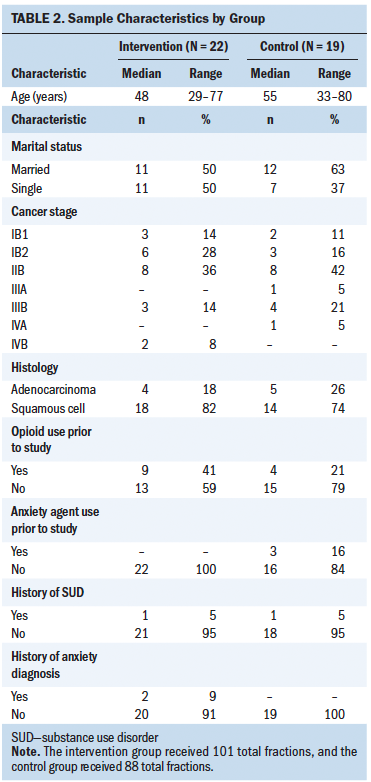
For both organism types, the one-day CFU means were less than the no-polish or four-day CFU means. For gram-positive organisms, tests for treatment differences found that the observed count for one-day-old polish was less than that of the unpolished nail (p = 0.04), and four-day-old polished nails had more microorganisms than the one-day-old polished nails (p = 0.03). Although gram-negative organisms demonstrated the same trends, the differences were not statistically significant (p = 0.3 and 0.17, respectively). For gram-negative organisms, there were fewer data to compare, because 51% (n = 45) of nurses had no gram-negative contamination on any of their nails.
Next, researchers wanted to study the relationship between the number of microorganisms and nail polish chipping. Chipping of the nail polish occurred almost immediately. When the polish was assessed by sample collection volunteers on day 1, 49% of participants had obvious nail polish chipping. By day 2, 90% of the sample had chipping, and by day 4, 100% had chipped polish. For gram-positive and gram-negative bacteria, there was a positive relationship between the age of the polish and amount of chipping. Table 3 shows the number of nails in each chip category as a function of bacteria type and polish age. 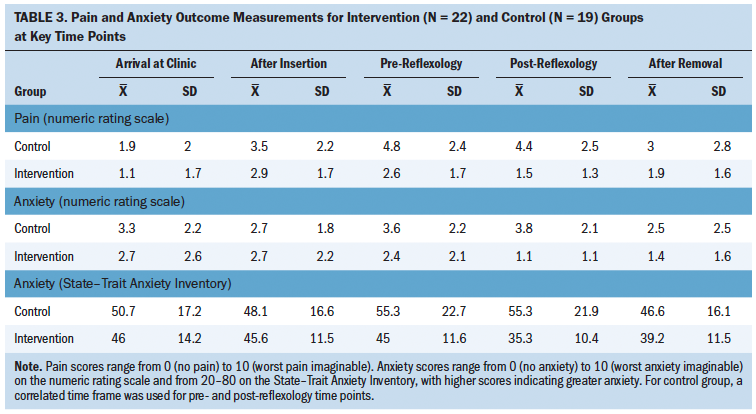
To assess the relationship between microorganism CFU count and the amount of polish chipping, day 1 data were tested for count differences between no chipping and minimal chipping, and day 4 data were tested for count differences between all categories of chipping. This analysis of the day 1 data found a statistically significant count difference for the gram-positive organisms (p = 0.0008) but not for the gram-negative organisms (p = 0.06). The model estimated mean count for the no-chipping group was 1,085 CFUs (95% confidence interval [CI] [419, 2,808]), and the mean count for the minimal-chipping group was estimated at 101 (95% CI [39, 265]).
The p values for gram-positive and gram-negative CFU count differences in different chipping conditions ranged from p < 0.01 to 0.06. For both bacteria types, the statistically significant comparisons were between the minimal-chipping group and all other chipping groups, and bacterial counts were increased as chipping increased. These trends are illustrated in Figure 4, which presents box plots of the number of CFUs for gram-positive and gram-negative organisms related to the amount of chipping. 
Discussion
In this study, it was determined that there were fewer bacteria on freshly polished nails with no chipping compared to unpolished nails. Although the authors did not explore any mechanistic antimicrobial variables, it appears that the presence of fresh polish confers some sort of protective effect against bacteria. The freshly polished nails were unchipped; however, they only remained unchipped for 24 hours or less. As the degree of chipping increased, so did the number of CFUs of gram-positive bacteria.
Previous studies were limited in size and setting, and this study seems to impart new findings. Arrowsmith and Taylor’s (2014) systematic review was limited to surgical staff using hand scrub rather than standard hand hygiene practice used outside of perioperative settings, making this study more generalizable to that nurse population. The current study confirms some findings of a study by Hewlett et al. (2018) that microorganism growth increases over time in all nail conditions but also shows a possible protective element if nail polish is unchipped.
Major strengths of the study include the rigorous methods applied in structuring all three nail conditions on the same nurse’s hand, which minimized bias. The decision to randomize different nails on the same nurse strengthens study results, because within-subject design reduces errors by exposing each participant to all treatments while reducing the effect of differences between factors that each participant is exposed to—in this case, handwashing efficacy, types of tasks performed, and frequency of washing hands. The no-polish, one-day-old polish, and four-day-old polish nails were all on the same hand of a nurse participant and, therefore, were exposed to the same type of cleaning and had the same potential for exposure to bacteria. Randomization of nails rather than participants may reduce conscious or unconscious participant bias.
Internal reliability was achieved by standardized manicurist training to a limited number (n = 12) of volunteers rather than allowing participants to apply their own polish. Sample collection was also done by only three study workgroup members who had demonstrated consistent collection technique. The participants, manicurists, sample collection volunteers, and laboratory personnel were all blinded to which nail was one-day-old polish and which was four-day-old polish. The investigators also looked at the degree of chipping in addition to simply the age of the polish.
Limitations
Limitations of the study include that, although a standard technique of polish application was chosen, not all polish formulations and application methods could be tested because of the overall cost (e.g., base coats, top coats, sticker-type nails, dip nails). An assumption may be drawn based on the entire body of evidence that nail coverings wear by either lifting (which causes increased risk of infection) or chipping, as shown in this study, no matter the formulation and application method. Also, the requirements of inpatient nursing schedules made it more difficult for inpatient nurses to be eligible to participate in the study. This resulted in the participants being more heavily represented by ambulatory staff. The difference in type of work performed by ambulatory versus inpatient nurses may be a confounding issue related to potential exposure to bacteria and transference of bacteria to patients.
Participant bias may have influenced hand hygiene practices in the form of the Hawthorne effect. The participants may have performed more effective hand hygiene because of awareness of being observed, but bacterial growth would be consistent in all three nails of each participant’s hand. Finally, nurses participating in the study did not have anonymity because of the obvious polish on two fingers of one hand and stickers worn on their clothing identifying them as research participants.
Implications for Nursing
This study presents an interesting conundrum for oncology nursing practice. On one side of the discussion, the data suggest that there is some level of protection against microorganisms provided by polish in the first 24 hours of wear. On the other hand, significant chipping starts quickly thereafter and progresses rapidly.
A crucial oncology nursing implication of this study relates to the care of neutropenic patients and finding interventions that protect vulnerable patients. Abou Dagher et al. (2017) found that hospitalized patients with cancer who became septic had a 2.32 times greater risk of dying. Therefore, infections are a serious concern for patients with cancer and the nurses who care for them. The current study suggests that the longer that nails have been polished, the more chipping occurs. Chipped polish is associated with higher levels of microorganisms carried on nurses’ hands. Oncology nurses’ duty to care compels them to take all reasonable measures to reduce the harm associated with cancer treatment, including providing the protection afforded by optimal hand hygiene practices.
Melnyk and Fineout-Overholt (2015) purport the use of the analogy of the three-legged stool when considering application of evidence-based practice in difficult situations. This metaphor encourages clinicians to use the best available research evidence in conjunction with clinical expertise while considering patients’ characteristics, values, and circumstances to inform care. The results of this study should be interpreted and applied to expert nursing practice in the care of vulnerable patient populations. Each institution and each practitioner should make their own decisions and interpretation of evidence into practice. More research is needed that tests these results and focuses on other evolving nail products. New studies should focus not only on the presence of bacteria but, if possible, also demonstrate how presence of bacteria on nails correlates with infection in patients.
This study is noteworthy because it investigates standard nail polish, which stands apart from other hand hygiene literature that focuses on artificial nails and gel polishes. Although the results may raise several personal and social questions about appearance, attire, and hygiene preferences and rights, they do provide a basis for nurses and others in healthcare leadership to initiate discussion about all nail coverings in their institutional policies. In addition, these results could have implications for other providers who have direct contact with patients, such as those in dentistry, medicine, emergency services, and other allied health fields. It will be imperative for oncology nurses and other healthcare providers to determine the best stance in relation to wearing nail polish and protecting patients with cancer. 
Conclusion
This study suggests that the bacterial load on nurses’ nails increases as nail polish chipping increases. For the authors’ institution, the optimal strategy to decrease the bioburden on direct patient care staff nails is to not wear polish when providing direct patient care. Although the study demonstrated that nail polish has a potentially protective element when compared to no polish, the length of protection is very short-lived (24 hours or less), and chipped polish increases microorganism growth quite significantly. Based on the evidence found regarding nail polish at the authors’ institution, the optimal strategy for preventing infection transmission from healthcare providers’ hands to patients with cancer is for direct care staff not to wear nail polish or other nail coverings.
About the Author(s)
Lisa Blackburn, MS, APRN-CNS-BC, AOCNS®, is a clinical nurse specialist, Kelly Acree, MPH, BSN, RN, CIC, is an infection preventionist, Judith Bartley, DNP, RN, SCRN, RN-BC, is a nurse educator, Elizabeth DiGiannantoni, BSN, RN, OCN®, is a STAT nurse, and Elizabeth Renner, BSN, RN, is a genitourinary/gynecologic oncology staff nurse, all at the Arthur G. James Cancer Hospital and Richard J. Solove Research Institute at the Ohio State University Comprehensive Cancer Center, and Loraine T. Sinnott, PhD, is a senior statistician in the College of Optometry at the Ohio State University, all in Columbus. No financial relationships to disclose. Blackburn, Bartley, DiGiannantoni, and Renner contributed to the conceptualization and design. Blackburn, Acree, Bartley, DiGiannantoni, and Renner completed the data collection. Renner and Sinnott provided statistical support. Blackburn, DiGiannantoni, Renner, and Sinnott provided the analysis. All authors contributed to the manuscript preparation. Blackburn can be reached at lisa.blackburn@osumc.edu, with copy to ONFEditor@ons.org. (Submitted September 2019. Accepted November 4, 2019.)
References
Abou Dagher, G., El Khuri, C., Chehadeh, A.A., Chami, A., Bachir, R., Zebian, D., & Bou Chebl, R. (2017). Are patients with cancer with sepsis and bacteraemia at a higher risk of mortality? A retrospective chart review of patients presenting to a tertiary care centre in Lebanon. BMJ Open, 7(3), e013502. https://doi.org/10.1136/bmjopen-2016-013502
Arrowsmith, V.A., & Taylor, R. (2014). Removal of nail polish and finger rings to prevent surgical infection. Cochrane Database of Systematic Reviews. Retrieved from https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003325.pub3…
Baumgardner, C.A., Maragos, C.S., Walz, J., & Larson, E. (1993). Effects of nail polish on microbial growth of fingernails. Dispelling sacred cows. AORN Journal, 58(1), 84–88. https://doi.org/10.1016/S0001-2092(07)65103-5
Boyce, J.M., & Pittet, D. (2002). Guideline for hand hygiene in health–care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR, 51(RR-16), 1–45.
Centers for Disease Control and Prevention. (2002). Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morbidity and Mortality Weekly Review, 51(16), 1-56.
Cimon, K., & Featherstone, R. (2017). Jewellery and nail polish worn by health care workers and the risk of infection transmission: A review of clinical evidence and guidelines. Canadian Agency for Drugs and Technologies in Health.
Coulliette, A.D., & Arduino, M.J. (2013). Hemodialysis and water quality. Seminars in Dialysis, 26(4), 427–438. https://doi.org/10.1111/sdi.12113
DeVita, V.T., Jr., Lawrence, T.S., & Rosenberg, S.A. (Eds.) (2015). DeVita, Hellman, and Rosenberg’s cancer: Principles and practice of oncology. Wolters Kluwer.
Gordin, F.M., Schultz, M.E., Huber, R., Zubairi, S., Stock, F., & Kariyil, J. (2007). A cluster of hemodialysis-related bacteremia linked to artificial nails. Infection Control and Hospital Epidemiology, 28(6), 743–744. https://doi.org/10.1086/517977
Gudiol, C., Aguado, J.M., & Carratalá, J. (2016). Bloodstream infections in patients with solid tumors. Virulence, 7(3), 298–308. https://doi.org/10.1080/21505594.2016.1141161
Hewlett, A.L., Hohenberger, H., Murphy, C.N., Helget, L., Hausmann, H., Lyden, E., Fey, P.D., & Hicks, R. (2018). Evaluation of the bacterial burden of gel nails, standard nail polish, and natural nails on the hands of healthcare workers. American Journal of Infection Control, 46(12), 1356–1359. https://doi.org/10.1016/j.ajic.2018.05.022
Johnson, L.A. (2014). Putting Evidence Into Practice: The process for evidence-based research. Clinical Journal of Oncology Nursing, 18(Suppl. 3), S2–S4. https://doi.org/10.1188/14.CJON.S3.2-4
Lingaas, E., & Fagernes, M. (2009). Development of a method to measure bacterial transfer from hands. Journal of Hospital Infection, 72(1), 43–49. https://doi.org/10.1016/j.jhin.2009.01.022
Manetti, M. (2012). Gel manicure cancer? UV drying technique may lead to health problems, study finds. Huffington Post. Retrieved from http://www.huffingtonpost.com/2012/03/06/gel-manicures-can-cause-cancer…
McClung, L., Obasi, C., Knobloch, M.J., & Safdar, N. (2017). Health care worker perspectives of their motivation to reduce health care-associated infections. American Journal of Infection Control, 45(10), 1064–1068. https://doi.org/10.1016/j.ajic.2017.05.002
McNeil, S.A., Foster, C.L., Hedderwick, S.A., & Kauffman, C.A. (2001). Effect of hand cleansing with antimicrobial soap or alcohol-based gel on microbial colonization of artificial fingernails worn by health care workers. Clinical Infectious Diseases, 32(3), 367–372. https://doi.org/10.1086/318488
Melnyk, B.M., & Fineout-Overholt, E. (2015). Evidence-based practice in nursing and healthcare: A guide to best practice (3rd ed.). Wolters Kluwer.
National Comprehensive Cancer Network. (2018). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prevention and treatment of cancer-related infections [v.1.2019]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf
Nuland, S.B. (2004). The doctor’s plague: Germs, childbed fever and the strange story of Ignác Semmelweis (pp. 100–102). W.W. Norton.
Pan, S.C., Chen, E., Tien, K.L., Hung, I.C., Shen, W.H., Chen, Y.C., & Chang, S.C. (2014). Assessing the thoroughness of hand hygiene: “Seeing is believing.” American Journal of Infection Control, 42(7), 799–801. https://doi.org/10.1016/j.ajic.2014.03.003
Pittet, D., Allegranzi, B., & Boyce, J. (2009). The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infection Control and Hospital Epidemiology, 30(7), 611–622. https://doi.org/10.1086/600379
Pittet, D., Allegranzi, B., Sax, H., Dharan, S., Pessoa-Silva, C.L., Donaldson, L., & Boyce, J.M. (2006). Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet. Infectious Diseases, 6(10), 641–652.
Pottinger, J., Burns, S., & Manske, C. (1989). Bacterial carriage by artificial versus natural nails. American Journal of Infection Control, 17(6), 340–344.
Schiffer, C.A., Mangu, P.B., Wade, J.C., Camp-Sorrell, D., Cope, D.G., El-Rayes, B.F., Gorman, M., Ligibel, J., Mansfield, P., & Levine, M. (2013). Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology, 31(10), 1357–1370. https://doi.org/10.1200/JCO.2012.45.5733
Siegel, J.D., Rhinehart, E., Jackson, M., & Chiarello, L. (2019). 2007 guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings. Retrieved from https://www.cdc.gov/infectioncontrol/pdf/guidelines/isolation-guideline…
Ulrich, N., Vonberg, R.P., & Gastmeier, P. (2017). Outbreaks caused by vancomycin-resistant Enterococcus faecium in hematology and oncology departments: A systematic review. Heliyon, 3(12), e00473. https://doi.org/10.1016/j.heliyon.2017.e00473
Wang, X.J., Lopez, S.E., & Chan, A. (2015). Economic burden of chemotherapy-induced febrile neutropenia in patients with lymphoma: A systematic review. Critical Reviews in Oncology/Hematology, 94(2), 201–212. https://doi.org/10.1016/j.critrevonc.2014.12.011
Wilson, B.J., Zitella, L.J., Erb, C.H., Foster, J., Peterson, M., & Wood, S.K. (2018). Prevention of infection: A systematic review of evidence-based practice interventions for management in patients with cancer. Clinical Journal of Oncology Nursing, 22(2), 157–168. https://doi.org/10.1188/18.CJON.157-168
Wynd, C.A., Samstag, D.E., & Lapp, A.M. (1994). Bacterial carriage on the fingernails of OR nurses. AORN Journal, 60(5), 799–805.




