Early Identification of Intracranial Hemorrhage Using a Predictive Nomogram
Objectives: To identify predictive signs and symptoms occurring in hospitalized adults with hematologic malignancies with intracranial hemorrhage (IH).
Sample & Setting: In a National Cancer Institute (NCI)–designated comprehensive cancer center, a retrospective matched case-control design included adult inpatients with hematologic malignancies with (n = 39) and without (n = 39) IH.
Methods & Variables: Conditional logistic regression, t test, and Fisher’s exact tests were used to assess increased risks for IH and the development of a prognostic nomogram with signs, symptoms, and laboratory values relevant to IH.
Results: Composite outcomes for signs, symptoms, and laboratory values were included in a prognostic nomogram that had good discriminative ability to predict IH, with a bootstrap corrected concordance index of 0.766 (95% confidence interval [0.657, 0.866]) and good calibration. Prognostic nomogram predicted patients with prolonged activated partial thromboplastin time (APTT) (greater than 30.6), headache, and systolic blood pressure (SBP) of 140 or greater were more likely to have IH.
Implications for Nursing: Nurses should recognize that patients with the combination of prolonged APTT, SBP of 140 or greater, and headache are more likely to have IH.
Jump to a section
A 72-year-old patient with newly diagnosed acute myelogenous leukemia (AML) on an inpatient hematologic malignancy unit had a delay in diagnosis of acute intracranial hemorrhage (IH) in February 2013. On day two of the patient’s chemotherapy regimen, the patient developed acute onset nausea, vomiting, headache, hypertension, and bradycardia. Although the signs and symptoms were communicated to the medical team, the head computed tomography (CT) scan demonstrating IH was not obtained until 12 hours later. By that time, the patient had developed mental status changes (a known symptom of IH) and incontinence. The patient was transferred to the neurology intensive care unit where he became agitated and aphasic. Several days later, he was transitioned to home hospice. The delay in diagnosis of IH and adverse outcome in this case served as the impetus to form an interprofessional team on the inpatient nursing unit to review patients diagnosed with IH.
IH is defined as any bleeding within the “intracranial vault, including the brain parenchyma and surrounding meningeal spaces” (Caceres & Goldstein, 2012, p. 771). This definition includes intracerebral hemorrhage, subarachnoid hemorrhage, and subdural hematoma. These hemorrhages usually occur suddenly from external or internal causes and can be life-threatening because the brain relies on blood vessels to supply oxygen and nutrients (Cleveland Clinic, 2016). As blood vessels in the brain leak or pool and put pressure on the brain, it becomes deprived of oxygen, which causes cell death (Cleveland Clinic, 2016). Where the injury is located affects what kind of deficits the patient may have. In adult patients with hematologic malignancies, IH is the leading cause of mortality after infection, with a mortality rate as high as 64%–67% within 30 days of IH diagnosis (Chen, Tai, Tsay, Chen, & Tien, 2009). Half of these deaths occur within the first two days; for those who survive, only 20% are expected to be functionally independent at six months (Broderick et al., 2007).
Several risk factors for IH in patients with cancer have been identified, including hypertension, low platelet count or platelet dysfunction, disseminated intravascular coagulation, sepsis, vessel wall abnormality, invasion or compression of vessels from a tumor in or adjacent to the brain, coagulation factor deficiency, and hyperleukocytosis, and incidence is higher in AML (Chen et al., 2012; Fang, Lin, & Ko, 2005). Profound thrombocytopenia and/or platelet dysfunction, one of the chief predisposing factors for IH in patients with hematologic malignancies, may be further exacerbated by treatment with antibiotics, tyrosine kinase inhibitors, and chemotherapy, as well as neutropenic fever and bacteremia/sepsis (Patel, Gojo, Tidwell, Sausville, & Baer, 2011). The presence of thrombocytopenia and other risk factors in patients with hematologic malignancies requires early recognition and communication of signs of IH crucial for rapid diagnosis.
However, initial signs and symptoms of acute and subacute IH are subtle, and symptoms, such as vomiting, headache, and nonspecific or nonfocal neurologic changes (e.g., somnolence), are also frequent side effects of chemotherapy alone, making diagnostic distinction challenging. Symptoms of IH can also differ depending on location of the bleed. IH is associated with general symptoms, including a change in level of consciousness; difficulty speaking, understanding, reading, or writing; loss of balance or coordination; difficulty with vision or swallowing; sudden and severe headache; and sudden tingling, weakness, numbness, or paralysis of the face, arm, or leg, particularly on one side of the body (Cleveland Clinic, 2016). More specifically, according to the American Heart Association and American Stroke Association guidelines, classical clinical presentation of intracerebral hemorrhage (ICH) includes “onset of a sudden focal neurological deficit which progresses over minutes to hours. . . . Headache is more common with ICH although less common in subarachnoid hemorrhage (SAH). Vomiting is more common with ICH than with either ischemic stroke or SAH” (Broderick et al., 2007, p. 2,002). Subdural hematomas “often follow obvious head trauma . . . producing impairment of consciousness and focal signs” (Campbell, 2005, p. 634). Increased blood pressure and decreased consciousness are also common with ICH (Broderick et al., 2007). However, limited research exists on the clinical manifestations of IH in patients with hematologic malignancies and early symptoms or signs that could aid in rapid diagnosis.
The purpose of this research was threefold. The first goal was to retrospectively quantify signs, symptoms, and risk factors that patients experienced up until the diagnosis of IH and compare them to matched controls to see if any cluster of signs, symptoms, or laboratory values consistently stood out in patients with IH. Second, the authors wanted to use those findings to create a predictive nomogram or tool for bedside nurses to identify patients who are at a higher risk of IH because they have a combination of known signs, symptoms, and risk factors for IH. Third, the authors hope to improve communication of these signs and symptoms between healthcare providers by providing nurses with the nomogram and confidence to speak up about patients at high risk for IH. If patients with IH can be identified earlier, an emergency head CT scan to establish the diagnosis could lead to earlier intervention and possibly improved clinical outcomes.
Methods
Participants and Setting
This retrospective study was conducted across four dedicated hematologic malignancy units, totaling 59 beds, at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital in Baltimore, Maryland. After receiving institutional review board expedited approval for a retrospective matched case-control study, medical records of patients with hematologic malignancy with IH from January 1, 2011, to December 31, 2014, were reviewed. Inclusion criteria were being aged 18 years or older, having a hematologic malignancy diagnosis, and receiving confirmation of IH diagnosis with radiographic imaging while admitted to the hospital. Sixty-one of 2,146 inpatients were identified to have IH in this time period using the following ICD9 codes: 430.0 (SAH) through 431 (ICH) through 432.9 (other and unspecified IH). IH was confirmed by review of CT scan or brain magnetic resonance imaging (MRI) reports. Twenty-two patients were diagnosed with IH prior to admission and were excluded from analysis because of insufficient laboratory data or lack of documented signs and symptoms. Data from patients with recurrent hemorrhages during their admissions were included only once, at the time of their first hemorrhage. For the 39 included patients with IH, the quality improvement staff members found 39 controls matched for diagnosis, treatment regimen, age, sex, and race in that order of priority.
Procedures
Once the 39 patients with IH and controls were identified, chart reviews began but differed slightly between patients with IH and controls. Some data were collected only at time of admission for all patients; other data were collected during length of stay (LOS) for controls or until day of IH for patients with IH. All patients had demographic data collected (i.e., race, age, sex, diagnosis, induction versus consolidation therapy, active versus non-active disease, and LOS). Date of admission, date of diagnosis, date of death (if applicable), day 1 of current chemotherapy regimen (if applicable), and history of hypertension or prior IH were also collected for patients with IH and controls. For patients with IH, the date of IH, time of CT scan, and death (if applicable) were collected.
Symptoms, such as presence of cough, number of days cough was present, presence of sudden onset vomiting, highest documented grade of vomiting, number of days headache was present, highest daily headache score, and neurologic changes, were obtained from day of admission through 24 hours after IH for patients with IH and through LOS for controls. Data collection was stopped for patients with IH at 24 hours after IH so that physiologic changes after IH did not obscure the collection of early predictors of IH. Documentation of falls was collected for patients with IH; if patients experienced a fall, the number of days the fall occurred prior to IH was recorded.
Signs collected included blood pressure, heart rate, and meeting systemic inflammatory response syndrome (SIRS) criteria. Number of days that systolic blood pressure (SBP) was 140 or greater or 160 or greater, that diastolic blood pressure (DBP) was 100 or greater, or that heart rate (HR) was less than 60 was obtained from admission through the day before IH for patients with IH and through LOS for controls. For SIRS criteria, white blood cell count (WBC) was omitted because most patients were neutropenic; therefore, any other two SIRS criteria for inclusion were used. SIRS criteria were obtained from admission through 24 hours after IH for patients with IH and through length of stay for controls.
Laboratory data collected on admission for all patients included WBC with percentage circulating blasts. In addition, average platelet count, average number of platelet transfusions, platelet count around lumbar puncture (if applicable), lumbar puncture date (if within one month of IH), history of or active central nervous system (CNS) disease, and presence of infection as defined by presence of positive blood or urine culture were also collected for patients with IH and controls. Other laboratory data that were collected from admission until the time IH was identified in patients with IH and through LOS for controls consisted of prothrombin time, activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrinogen, and number of days platelet count was less than 20,000.
Chest CT scans were reviewed for patients with IH from admission until 24 hours after IH diagnosis or through LOS for controls to capture active pneumonia. Medication review of all patients included presence of vasopressors, antihypertensive medications, anticoagulant medications, or tyrosine kinase inhibitors from admission through diagnosis of IH for patients with IH or LOS for controls.
Statistical Analyses
Clinical characteristics measured at baseline and summary measures from the inpatient hospital stay were compared between patients with IH and matched controls using exact McNemar’s tests for categorical measures and Wilcoxon signed rank tests for continuous measures. Assessment and vital sign data were collected from the date of admission to the date of the IH plus 24 hours. For each matched control, the exposure period for measuring these assessments and vital signs were limited to the time period as the corresponding matched case. For example, if an included patient experienced IH five days after hospital admission, the authors used only data collected during the first five days of the matched control patient’s hospital admission in the analyses. The presence of risk factors at any time during the exposure period was included as a potential predictor in logistic regression models with generalized estimating equations while considering each matched case/control pair as the clustering unit. This approach allowed the authors to estimate predicted probabilities from the model for developing the prognostic nomogram. Odds ratios from the models represent the relative changes in the risk of IH with the presence of a risk factor. Analyses were completed using R, version 3.2.2.
Prognostic Nomogram Development
A multivariable predictive model for having IH was developed based on examination of univariate logistic regression analyses using the factors described previously. When evaluating the univariate analyses, the current authors also considered the correlation between risk factors and avoided including any pairs together in the multivariable model (e.g., nausea and vomiting often appear together). The authors selected no more than three signs, symptoms, or laboratories for the multivariable model, guided by the recommendation that the number of predictors in the nomogram should generally be no more than 10% of the smaller of the number of patients with IH and number of controls (Harrell, 2001). The final multivariable model was selected based on the area under the curve (AUC) and the receiver operating characteristic (ROC), or concordance index, which represents the probability of correctly discriminating patients with IH from controls using the predicted probabilities from that model. An AUC of 1 represents perfect discrimination, and 0.5 represents a random assignment or guessing. The final model was internally validated using a bootstrap approach for estimating the corrected concordance index and its corresponding 95% confidence interval (CI). The calibration of the final model was examined using a plot of the observed to predict probabilities that divided patients into four groups based on the distribution of the predicted probabilities.
Results
Sample Characteristics
From January 1, 2011, to December 31, 2014, the incidence of IH in the patient population with hematologic malignancies was 2.8% (61 of 2,146 unique patient admissions) at the comprehensive cancer center. Thirty-nine patients met inclusion criteria, and 39 matched controls were identified. Patients with IH and controls were closely matched for age (mean age was 53 years), diagnosis, phase of treatment (induction versus consolidation), sex, and race (see Table 1). AML was the most common diagnosis (n = 22 versus n = 24 for patients with IH and controls, respectively), and patients were most frequently undergoing induction chemotherapy (n = 27 for patients with IH, n = 31 for controls). Hyperleukocytosis (WBC greater than 100,000) was present equally (n = 3 in both groups). Having WBC greater than 30,000 was slightly higher in controls (n = 10) than in patients with IH (n = 9). Prior history of hypertension and presence of active disease on admission were also equally distributed between the groups (see Table 2). Among 22 excluded patients, 9 were female, and the most common diagnoses were AML, chronic myeloid leukemia, and acute lymphoblastic leukemia. Leukocytosis existed in 3 of 22 of the excluded patients with IH. 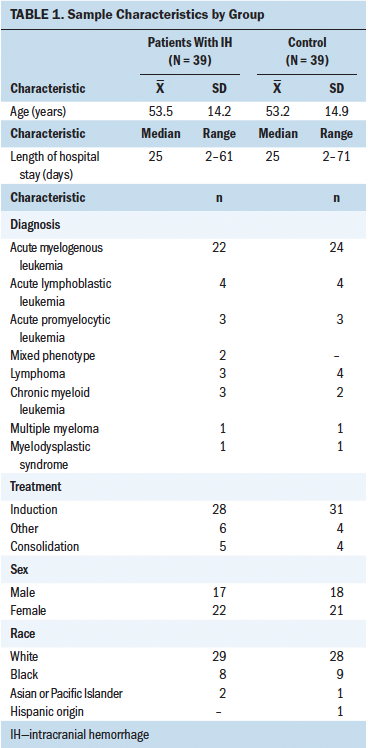
The authors confirmed that 26 of 39 patients with IH included in the study were deceased as of April 2015. These 26 patients lived an average of 93 days after IH was diagnosed, with a range of 3 hours to 590 days. Of the hemorrhages found in all patients with IH, 33 of 50 were extra-axial (epidural, subdural, or subarachnoid), and 17 of 50 were intra-axial (intraparenchymal or intraventricular). More specifically, 20 of 50 of patients with IH had subdural hematomas (SDHs), 14 of 50 had intraparenchymal bleeding, 13 of 50 had subarachnoid bleeding, and 3 of 50 had intraventricular bleeding. The relative predominance of SDH confirms findings by Reichman et al. (2012) that leukemia is frequently associated with SDH. Patients had bleeds in multiple sites.
[[{"type":"media","view_mode":"media_original","fid":"40006","attributes":{"alt":"","class":"media-image","height":"765","typeof":"foaf:Image","width":"624"}}]]
Variations in Signs and Symptoms
Some signs and symptoms were more frequently encountered in patients with IH than in controls, such as having fallen within 30 days prior to IH; presence of cough, nausea, vomiting, or headache; meeting SIRS criteria; SBP of 140 or greater or 160 or greater; and DBP of 100 or greater. With the exception of neurologic changes (p = 0.009), none of the differences reached statistical significance. Although 4 of 39 patients with IH had a documented fall within one month prior to IH, none had fallen the day of IH. All of the falls occurred prior to the patients being admitted to the hospital. Three of the four falls were unwitnessed, so only one patient was known to have hit her head upon falling.
With regard to specific neurologic changes, the most common symptoms for patients with IH and controls included nonfocal alterations, such as delirium, confusion, drowsiness, and disorientation. However, changes in vision or pupils, including diplopia, nystagmus, blurry vision, and unequal pupil size, were present in 13 patients with IH and only 1 control. Unresponsiveness was also more common in patients with IH (n = 7) than in controls (n = 1). Lethargy (n = 4), peripheral neuropathy (n = 3), and facial numbness (n = 2) were found more often in controls than in patients with IH. Some neurologic changes were unique to the patients with IH and consisted of difficulty finding words, seizures, inappropriate response to command, disorganized speech, slurred speech, hearing loss, obtundation, eye deviation, Bell’s palsy, lack of cough reflex, difficulty swallowing, and difficulty walking/unsteady gait. Average time to head CT/MRI from onset of first neurologic sign or symptom was 56 hours, with a range of 36 minutes to 207 hours (excluding incidental findings).
Variations in Laboratory Values and Radiology Tests
The frequency of laboratory abnormalities and/or radiologic procedures and findings was similarly distributed between patients with IH and controls, but some abnormalities appeared to occur more commonly in patients with IH, such as CNS disease, lumbar puncture within 30 days prior to IH, active pneumonia (during admission based on radiologic results), fibrinogen of less than 150 or greater than 450, and INR of greater than 1.5. However, only the average number of platelet transfusions was statistically significantly higher (median difference = 0.29, 95% CI [0.15, 0.43], p < 0.001), and average platelet count was statistically significantly lower in patients with IH (median difference = –12, 95% CI [–23.5, –2.5], p = 0.02).
[[{"type":"media","view_mode":"media_original","fid":"40011","attributes":{"alt":"","class":"media-image","height":"496","typeof":"foaf:Image","width":"623"}}]]
The percentages of patients with IH and controls that experienced at least one potential sign, symptom, or laboratory value of IH are shown in Figure 1. The univariate regression models identified having APTT greater than 30.6, SBP greater than or equal to 140, and headache as predictors of IH (see Table 3). All other signs, symptoms, and laboratory values except for heart rate of less than 60 were associated with IH but were not statistically significant. Anticoagulant use was similar between patients with IH (n = 7 of 39 patients) and controls (n = 6 of 39 patients).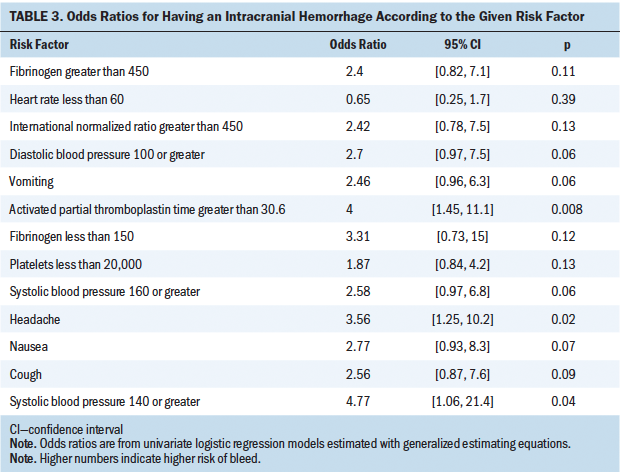
Multivariate Analysis and Nomogram
The final multivariable logistic regression model for predicting IH included the following: APTT greater than 30.6, SBP of 140 or greater, and headache (see Table 4). The concordance index for this predictive model is 0.763. After an internal validation of the model using a bootstrap approach with 2,500 simulations, the corrected concordance index was 0.766 (95% CI [0.657, 0.866]). An examination of the predicted probabilities against the observed proportion of patients with IH in the calibration plot shows very good model calibration.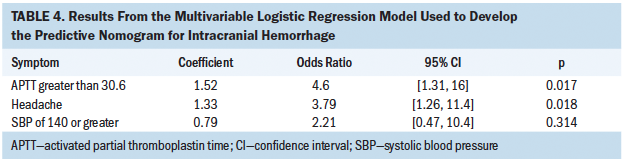
Discussion
Symptoms
This retrospective analysis substantiates findings of other investigators and allows the development of a potentially predictive model for early recognition and intervention for IH. Although headache is a known symptom of IH, the current research showed that the frequency of headache was significantly higher in patients with IH. Severity of headache did not differ between patients with IH and controls. Hospitalized patients with hematologic malignancy can also experience vomiting induced by chemotherapy or antibiotics, but it occurs more frequently in patients with IH and should be considered as either a precipitating factor or a possible symptom of CNS bleeding. Patients with cough should be monitored for other signs of IH because it was a more common symptom in patients with IH than in controls in this study. Having had a fall places patients at higher risk for IH, so ensuring that fall-reduction strategies are being used is important. In the hematologic malignancy population, routine neurologic assessments should include assessing the patient’s pupils, vision, and speech because these are associated with IH.
Signs
This study confirmed hypertension as a critical risk factor for IH (Hu, Wang, & Luo, 2013). In the hematologic malignancy population, hypertension is not always aggressively controlled because of fear of sepsis-related hypotension. However, hypertension should not be ignored in this patient population either. Although not statistically significant, meeting SIRS criteria was also more common in patients with IH. These patients should be closely monitored for additional signs and symptoms of IH.
Laboratory Values and Radiographic Tests
Several laboratory abnormalities were more common in patients with IH. Presence of CNS disease and intrathecal chemotherapy have been previously recognized as risk factors for IH, but those factors did not reach statistical significance in the current study because of low number of participants (Reichman et al., 2012; Ryu et al., 2016). Patients with CNS disease or recent lumbar punctures should be closely monitored for additional signs and symptoms of IH. In addition, the current authors confirmed that prolonged APTT was associated with IH. Routinely checking coagulation parameters, particularly APTT in the hematologic malignancy population, may help identify patients at higher risk for bleeding. Patients with IH also had a lower median platelet count and received more platelet transfusions compared to those without IH, as reported previously (Ryu et al., 2016). Although requirements for more transfusions prior to IH may be, in part, because of lower platelet counts, it is important to monitor patients who may be refractory to transfusions or need additional transfusions because of active bleeding.
Limitations
Limitations of this study included being a retrospective review from only one institution and having a small study population. Asymptomatic, self-limited bleeding may have been missed in patients who did not have a CT scan. Not all patients with IH had undergone lumbar punctures or postmortem autopsies; therefore, it is unknown how many had CNS involvement by their underlying disease. A new computer documentation system implemented in November 2011 resulted in a change in assessment of nausea and vomiting from the Rhodes Scale to the National Cancer Institute scale, which, in turn, may have introduced some inconsistency in quantitating the severity of nausea and vomiting. Although all nurses involved in study were trained to mine the charts for data in the same way, there may have been inconsistencies or human error in data collection or transfer of data into the spreadsheet. Because it is difficult to know the precise onset of IH in most cases, vital sign and assessment changes could have been a result of the IH rather than a precursor or risk factor. Limitations regarding the development of the predictive nomogram include a small sample size. A larger sample of patients would allow the authors to explore more potential risk factors to include in the nomogram and to assess the model calibration with a higher number of observed to predicted probability estimates.
Implications for Nursing Practice
Many patients with hematologic malignancy have multiple risk factors for IH, including meeting SIRS criteria; having hypertension, CNS involvement or recent lumbar punctures, prolonged APTT, or thrombocytopenia; and requiring frequent platelet transfusions. Many also display early symptoms of IH, including headaches, vomiting, cough, and neurologic changes. It can be difficult to determine whether a patient is having side effects from chemotherapy or experiencing a more serious complication like IH. The current authors’ research discovered that the combination of SBP of 140 or greater, APTT greater than 30.6, and headache are likely predictors of IH in this population.
However, numerous risk factors and symptoms of IH exist that should not be overlooked. The current research also confirmed neurologic changes, specifically changes in pupils, vision, or speech, should be closely monitored, particularly in the presence of hypertension, headache, and coagulopathies. Patients with frequent, but not necessarily severe, headaches should also be closely monitored for IH. Communicating signs of hypertension to the healthcare team is equally important. Laboratory tests for APTT should be routinely drawn and monitored in these patients, with abnormal results immediately communicated to a medical provider. Proactive management of hypertension and coagulopathies could possibly decrease the incidence of IH in the population of adult patients with hematologic malignancies, but that was not examined in this study.
Providing staff nurses with education on the importance of assessing headache, hypertension, and prolonged APTT is important because these are known predictors of IH in this patient population. Ideally, this would be part of the routine nursing assessment once the nomogram has been validated. Communication of these predictors of IH to the healthcare team would also be crucial in expediting diagnosis. In addition, the following individual signs, symptoms, and laboratory values should be closely monitored because they alone can be signs of IH or put patients at higher risk for IH:
• Any neurologic changes
• Experienced a fall
• CNS disease
• Cough
• Active pneumonia or vomiting
• Meet SIRS criteria
• Recent lumbar puncture (within past 30 days)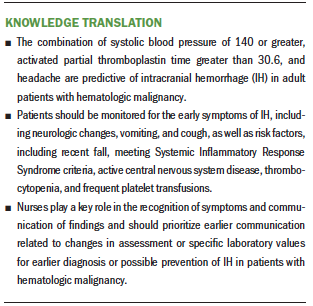
Conclusion
The prognostic nomogram developed in this study identified that the combination of headache, hypertension (SBP of 140 or greater), and prolonged APTT were predictive of having IH in the adult inpatients with hematologic malignancies. The authors plan to test this nomogram in a prospective study with a larger sample size to validate the findings. A larger sample size may need to be used to make a more inclusive nomogram because many known signs and symptoms of IH should not be overlooked. Using the nomogram, the authors anticipate reducing the time from onset of IH signs and symptoms to the institution of appropriate evaluation and management by decreasing the time to head CT for confirmation of IH.
The authors gratefully acknowledge Hetty Carraway, MD, for project design.
About the Author(s)
Frances Leah Chandler, MS, RN, OCN®, is an RN, and Joyce Kane, MSN, RN, CPHQ, is a quality improvement safety team leader, both in the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital in Baltimore, MD; Amanda L. Blackford, ScM, is a principal biostatistician in the Division of Biostatistics and Bioinformatics at Johns Hopkins University in Baltimore; Margaux Weinberger, BSN, RN, is a labor, delivery, recovery, and postpartum RN at the Swedish Medical Center in Seattle, WA; Kavitha C. Wagner, BSN, MSN, OCN®, CRNP, is a nurse practitioner for Stella Maris in Lutherville-Timonium, MD; Ivana Gojo, MD, is an associate professor of oncology in the School of Medicine at Johns Hopkins University; and Melanie Cohen, BSN, RN, is a nurse clinician III, and Colleen Apostol, MSN, RN, OCN®, is a nurse manager, both in the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital. This research was funded by the 2013 Jennifer L. Brager Memorial Award for New Cancer Research. Gojo is supported by funding from Amgen and Merck and has previously received honorarium for participation in advisory or review activities from Amgen, Merck, and Novartis. Chandler, Kane, Gojo, Cohen, and Apostol contributed to the conceptualization and design. Chandler, Kane, Weinberger, Wagner, and Cohen completed the data collection. Blackford, Weinberger, and Gojo provided statistical support. Chandler, Kane, Blackford, and Gojo provided the analysis. Chandler, Blackford, and Gojo contributed to the manuscript preparation. Chandler can be reached at lkerr2@jhmi.edu, with copy to ONFEditor@ons.org. (Submitted June 2017. Accepted September 19, 2017.)
References
Broderick, J., Connolly, S., Feldmann, E., Hanley, D., Kase, C., Krieger, D., . . . Zuccarello, M. (2007). Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: A guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke, 38, 2001–2023. https://doi.org/10.1161/STROKEAHA.107.183689
Caceres, J.A., & Goldstein, J.N. (2012). Intracranial hemorrhage. Emergency Medicine Clinics of North America, 30, 771–794. https://doi.org/10.1016/j.emc.2012.06.003
Campbell, W.W. (2005). DeJong’s the neurologic examination (6th ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
Chen, C.Y., Tai, C.H., Cheng, A., Wu, H.C., Tsay, W., Liu, J.H., . . . Tien, H.F. (2012). Intracranial hemorrhage in adult patients with hematological malignancies. BMC Medicine, 10, 97. https://doi.org/10.1186/1741-7015-10-97
Chen, C.Y., Tai, C.H., Tsay, W., Chen, P.Y., & Tien, H.F. (2009). Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia. Annals of Oncology, 20, 1100–1104. https://doi.org/10.1093/annonc/mdn755
Cleveland Clinic. (2016). Intracranial hemorrhage, cerebral hemorrhage, and hemorrhagic stroke. Retrieved from http://my.clevelandclinic.org/services/neurological_institute/cerebrova…
Fang, H.Y., Lin, C.Y., & Ko, W.J. (2005). Hematology and coagulation parameters predict outcome in Taiwanese patients with spontaneous intracerebral hemorrhage. European Journal of Neurology, 12, 226–232. https://doi.org/10.1111/j.1468-1331.2004.01018.x
Harrell, F.E. (2001). Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag.
Hu, Y.Z., Wang, J.W., & Luo, B.Y. (2013). Epidemiological and clinical characteristics of 266 cases of intracerebral hemorrhage in Hangzhou, China. Journal of Zhejiang University, 14, 496–504. https://doi.org/10.1631/jzus.B1200332
Patel, S.B., Gojo, I., Tidwell, M.L., Sausville, E.A., & Baer, M.R. (2011). Subdural hematomas in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia receiving imatinib mesylate in conjunction with systemic and intrathecal chemotherapy. Leukemia and Lymphoma, 52, 1211–1214. https://doi.org/10.3109/10428194.2011.566950
Reichman, J., Singer, S., Navi, B., Reiner, A., Panageas, K., Gutin, P.H., & DeAngelis, L.M. (2012). Subdural hematoma in patients with cancer. Neurosurgery, 71, 74–79. https://doi.org/10.1227/NEU.0b013e3182517938
Ryu, J.A., Lee, D., Yang, J.H., Chung, C.R., Park, C.M., Suh, G.Y., & Jeon, K. (2016). Spontaneous intracranial haemorrhage in critically ill patients with malignancies. Supportive Care in Cancer, 24, 2971–2978. https://doi.org/10.1007/s00520-016-3094-5




