Distress, Fatigue, and Sexuality: Understanding and Treating Concerns and Symptoms in Patients With Multiple Myeloma
Background: The psychological needs of patients and caregivers may be inadvertently overlooked, contributing to the patient’s distress and possibly compromising outcomes. Untreated, these psychological needs may impair the patient’s ability to make decisions and adhere to treatment.
Objectives: This article aims to present consensus statements to guide oncology nurses in the recognition and management of distress, fatigue, and sexual dysfunction in patients with multiple myeloma (MM).
Methods: Members of the International Myeloma Foundation Nursing Leadership Board reviewed the current literature and clinical experience regarding interventions related to distress, fatigue, and sexual dysfunction in patients with MM.
Findings: Ongoing patient education and attention to medical and psychological care is important to assess and address patients’ needs, such as cancer-related fatigue, sexual dysfunction, and distress.
Jump to a section
Across ambulatory and inpatient settings, an estimated 47% of patients with cancer have a psychiatric disorder, 68% of which are classified as an adjustment disorder related to some aspect of the cancer illness (Derogatis et al., 1983). The true incidence of psychiatric disorders is unknown because healthcare providers do not routinely assess patients for them. With complex cancer therapies moving away from inpatient to ambulatory settings, identifying psychiatric disorders has become more problematic.
In 1997, a multidisciplinary panel of the National Comprehensive Cancer Network (NCCN) developed consensus guidelines for treating the emotional and psychological effects of cancer (Holland & Bultz, 2007). The panel concluded that the term distress was less stigmatizing and more acceptable to patients and oncologists than psychological, psychiatric, or emotional disorder. The term continues to lack a clear definition despite wide use (Phillips, 2009).
The Institute of Medicine identified quality psychological care as a vital component of comprehensive cancer care (Adler & Page, 2008). In collaboration with the NCCN panel, the American College of Surgeons (2012) Commission on Cancer developed a new accreditation standard for assessing and treating the psychological concerns of patients with cancer. Cancer centers seeking accreditation are required to assess these concerns at least once during cancer treatment.
Distress in Patients With Multiple Myeloma
Stress is defined as a feeling of being overwhelmed, worried, or run down as a result of responses to one’s internal or external environment (Schneiderman, Ironson, & Siegel, 2005). When these responses are no longer effective in maintaining health or are counterproductive, stress can become distress (Weisman & Worden, 1977).
Distress, however, is more difficult to define than stress (Horwitz, 2007; Phillips, 2009). It consists of a collection of symptoms and not a specific diagnosis and is closely associated with many psychiatric disorders. Distress-related functional impairment in patients without a clinical psychiatric disorder (non-disordered people) and individual psychopathology are often treated as mental disorders (Horwitz, 2007; Phillips, 2009). One point of view is that distress arises in non-disordered people when psychological mechanisms allow them to respond appropriately to stressful circumstances, whereas mental disorders reflect dysfunctional and perhaps deeper internal mechanisms that create problems for affected individuals and those around them (Horwitz, 2007). In any case, distress and psychopathology affect several dimensions of quality of life, which independently predicts overall survival, making mental health assessments an important part of cancer care (Strasser-Weippl & Ludwig, 2008).
Unmet Mental Health Needs
The most prevalent patient needs in cancer care are the lack of disease-related information and social and psychological support (Husson et al., 2013; Lamers et al., 2013; Swash, Hulbert-Williams, & Bramwell, 2014; Zabora et al., 2015). The experience of disease-related distress depends not only on the severity and frequency of its symptoms, but also on the meanings and expectations that patients attach to their symptoms (Husson et al., 2013). Therefore, ongoing patient education and attention to medical and psychological care is fundamentally important to reducing disease burden (Sherman, Simonton, Latif, Spohn, & Tricot, 2004).
In patients with multiple myeloma (MM), unmet mental health needs appear to be the highest and most varied during treatment. Some patients express these needs at diagnosis, but most only after treatment (Swash et al., 2014). Although many patients accept treatment and are satisfied with the physical aspects of their care, many are reluctant to discuss their feelings and fears with their healthcare team, particularly if providers appear to be busy. Willingness of healthcare professionals to listen is one of the most positive and helpful aspects of patient care. Assessing the need for psychosocial care is paramount in all patients with cancer, particularly in the early phases of diagnosis and treatment, but the best time for such assessment may vary according to disease stage (Harrison, Young, Price, Butow, & Solomon, 2009). For example, a mental health assessment should be a priority if the disease relapses or requires a change in therapy (Cormican & Dowling, 2016; Maher & de Vries, 2011).
Studies have reported that patients with MM believe their cancer is rarer than other malignancies (Kelly & Dowling, 2011). Many patients had never heard of the disease before their diagnosis (Stephens, McKenzie, & Jordens, 2014). Providing information about MM and its personal and social implications is important but often does not occur. The causes and risk factors of MM are unknown, and information on symptom management is often inconsistent. This uncertainty contributes to distress. In addition, family members are often shocked by the diagnosis and by the patient’s frequent encounters with physicians for treatment of chronic pain, infections, and bone fractures (Vlossak & Fitch, 2008).
Many studies have evaluated treatment distress in MM (Boland et al., 2014; Dahan & Auerbach, 2006; Kelly & Dowling, 2011; Potrata, Cavet, Blair, Howe, & Molassiotis, 2010, 2011; Trask et al., 2002). For example, patients have referred to the period of treatment as “looking dead” because of the difficulties in eating and weight loss (Potrata et al., 2010). However, patients report that toxicity was acceptable and quality of life was good three or more months post-transplantation (Olivieri et al., 2001). A considerable amount of patients with MM may experience several distressing symptoms before transplantation and need more immediate, intensive, and supportive care. In time, patients learn their limitations and develop strategies for managing fatigue, distress, pain, and neuropathy as best they can (Stephens et al., 2014).
Assessment and Interventions
The first published clinical practice guidelines for managing distress in patients with cancer were released in 1999 by the NCCN and provided a framework for assessing and managing distress (Holland, Greenberg, & Hughes, 2006). Screening tools, such as the Distress Thermometer and NCCN Clinical Practice Guidelines in Oncology for Distress Management, may help patients and their caregivers adjust to the stresses of living with MM. Patients with MM are generally eager to accept psychological interventions, such as relaxation techniques, psychological counseling, and peer-support groups, when provided the opportunity, even if they do not ask for them (Lamers et al., 2013).
For mild to moderate distress, interventions include cognitive behavioral therapy, psychotherapy, and medications. Exercise can be beneficial to manage distress symptoms (Holland & Alici, 2010). The results of a meta-analysis found that yoga had little benefit in managing symptoms in patients with hematologic cancers, including MM (Felbel, Meerpohl, Monsef, Engert, & Skoetz, 2014). If corticosteroids, often used with MM, are causing moderate to severe mood swings, reviewing disease status and adjusting the dosage of corticosteroids may be warranted (King & Faiman, 2017). Anxiolytics and antidepressants can be effective in treating anxiety and distress, but their side effect profiles must also be considered (Jacobsen, Donovan, Swaine, & Watson, 2006; Pirl, 2004; Williams & Dale, 2006). Access to social workers or psychologists for cognitive behavioral therapy, psychotherapy, and medication management may be limited, but patients with moderate to severe distress should be evaluated immediately by a trained psychiatric practitioner when possible (see Figure 1). 
Evidence-Based Recommendations for Distress
Level of Evidence I
• The International Myeloma Foundation (IMF) Nurse Leadership Board (NLB) recommends screening for distress among all patients with MM. Distress and psychopathology affect several dimensions of quality of life, which independently predicts overall survival (NCCN, 2017b; Strasser-Weippl & Ludwig, 2008).
• Interventions, such as listening to the patient’s concerns and offering cognitive behavioral therapy, may reduce distress among patients with cancer (Maher & de Vries, 2011; NCCN, 2017b).
• Patients should be made aware of the value of relaxation techniques, psychological counseling, and peer-support groups (Lamers et al., 2013).
Cancer-Related Fatigue in Patients With Multiple Myeloma
Cancer-related fatigue (CRF) is a “distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment” (NCCN, 2017a, p. FT-1). Compared to fatigue in healthy individuals, CRF is more severe and more distressing and tends to be resistant to rest. Despite being strongly related to quality of life, CRF is often underrecognized and undertreated (Mitchell, 2011). Healthcare providers may not appreciate the implications of fatigue (Mortimer et al., 2010) (see Figure 2), and patient communication with clinicians about fatigue is often inadequate. Patients may be reluctant to discuss their fatigue because they perceive it as untreatable or are unaware of treatment options. They may be reluctant to take additional medications or are concerned that they will be seen as complaining (Horneber, Fischer, Dimeo, Rüffer, & Weis, 2012; Mitchell, 2011) (see Figure 3).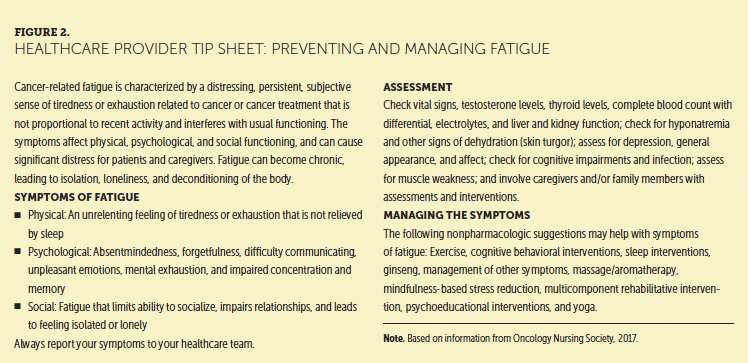
Characteristics
CRF is a personal experience, so its characteristics are often subjective and vary by individual. Some patients describe mental fogginess, inertia, or loss of efficiency, whereas others may describe it as an excessive need to rest, the inability to recover promptly from exertion, or muscle heaviness and weakness (Mitchell, 2011). Disruptive symptoms of chronic fatigue may persist for months to years after cancer therapy is completed (Horneber et al., 2012).
At least two dimensions of CRF have been described: mental and physical (Horneber et al., 2012). Mental fatigue includes difficulties with cognition, concentration, and speed of information processing. Patients with mental fatigue may have difficulty understanding simple instructions or completing a to-do list. They may describe absentmindedness, forgetting important appointments or to take medications, or difficulties in communicating with healthcare providers and family members. Patients may also report negative or unpleasant emotions, mental exhaustion, and impaired concentration and memory (Mitchell, 2011).
[[{"type":"media","view_mode":"media_original","fid":"36031","field_deltas":{"1":{}},"link_text":null,"fields":{},"attributes":{"height":"612","width":"364","class":"media-image media-element file-media-original","data-delta":"1"}}]]
Symptoms of physical fatigue include exhaustion, weakness, and tiredness (Horneber et al., 2012). Patients may report having heavy limbs or feeling slow, weary, sluggish, or unable to carry out activities of daily living. Patients commonly report limited energy and an inability to complete routine tasks, such as housecleaning, cooking, or recreational hobbies. Even routine activities, such as getting dressed, require more effort than usual. Mental and physical fatigue may prevent patients from participating in relationships and limit socialization, leading to isolation and loneliness (NCCN, 2017a).
Causes
Contributors to CRF in MM are multidimensional. They are thought to involve anemia, pain, reduced activity, insomnia, therapy toxicity, and myeloid suppression (Coleman et al., 2011; Smith et al., 2015; Stone & Minton, 2008). Other contributors include infection, malnutrition, cachexia, and medication side effects. Hypothyroidism and cardiac, pulmonary, hepatic, and renal impairment can contribute to profound fatigue, which can become more severe during cancer treatment (Mitchell, 2011). Fatigue is also commonly reported after autologous stem cell transplantation (Miceli et al., 2013).
Patients with MM are at risk for treatment-related fatigue, which can be short- or long-term (Miceli et al., 2013). Immunomodulators, such as thalidomide, lenalidomide, and pomalidomide, are associated with marked fatigue, as are proteasome inhibitors, such as bortezomib and carfilzomib (Amgen, 2017; Celgene, 2016, 2017a, 2017b; Millennium Pharmaceuticals, 2017). In addition, opioids, hypnotics, anxiolytics, antihistamines, antiemetics, anticonvulsants, antihypertensives, and insomnia medications can cause medication-related fatigue and enhance the symptoms of fatigue from other causes. Corticosteroids, such as dexamethasone and prednisone, carry well-known neuropsychological effects that can lead to anxiety, distress, insomnia, mood swings, depression, and fatigue. Side effects of corticosteroids, many of which contribute to CRF, are discussed in Figure 4. Although steroids are associated with improved energy, this experience is followed by intense fatigue, called a “let-down effect,” for several days after steroid administration (King & Faiman, 2017). Steroids affect numerous body systems. Figure 5 provides a patient education tool for continuing treatment after corticosteroid side effects. 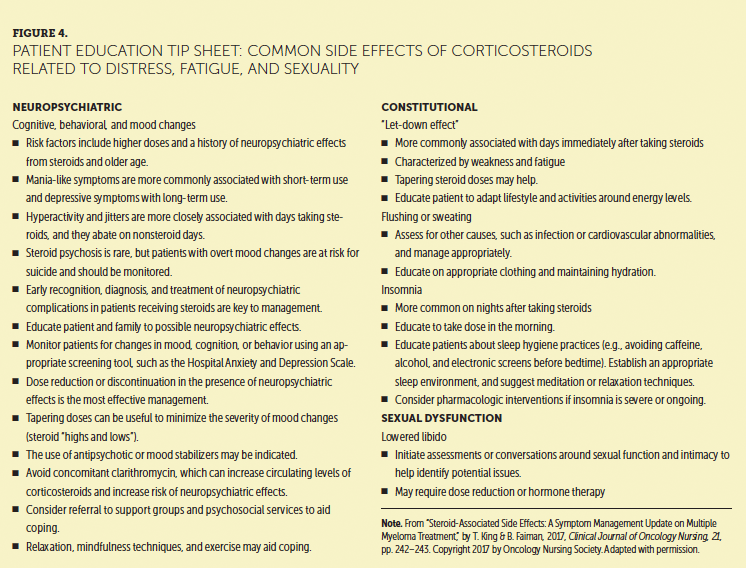
Assessment
All patients should be assessed for CRF. The NCCN (2017a) strongly encourages healthcare providers to assess fatigue at baseline and with every major change in the patient’s care or health. CRF assessments include identifying its characteristics, its impact on quality of life, and any potential contributing factors (Mitchell, 2011). Several validated instruments are widely used to diagnose fatigue in patients with cancer, including the Functional Assessment of Cancer Therapy, Brief Fatigue Inventory, and the Fatigue Symptom Inventory (NCCN, 2017a).
[[{"type":"media","view_mode":"media_original","fid":"36036","field_deltas":{"2":{}},"link_text":null,"fields":{},"attributes":{"height":"454","width":"747","class":"media-image media-element file-media-original","data-delta":"2"}}]]
Physical examination can determine contributing factors that lead to fatigue (Mitchell, 2011; NCCN, 2017a). For example, constitutional signs might identify nutritional deficiencies, whereas vital signs might indicate infection. A general assessment of appearance, mood, and manner may reveal anxiety or depression. Assessing the musculoskeletal system might reveal focal or generalized weakness, muscle mass loss, joint pain, warm or edematous joints, muscle pain, muscle twitching, limitations in range of motion, or bone pain. Abnormal skin turgor may indicate that dehydration could be contributing to CRF. Decreased or adventitious lung sounds might indicate pulmonary edema, chronic obstructive pulmonary disease, or pneumonia that reduces tolerance to activity. Abnormal heart sounds and jugular venous distension might indicate that congestive heart failure is causing fatigue (Mitchell, 2011).
Diagnosing the underlying cause of fatigue in patients with cancer is problematic because no diagnostic test is specific for it (Howell et al., 2013). However, reviewing medications, obtaining laboratory tests (e.g., complete blood counts with differential; electrolytes; renal, liver, and thyroid function tests) may help determine the cause of fatigue. Table 1 provides a framework to assess CRF in patients with MM. 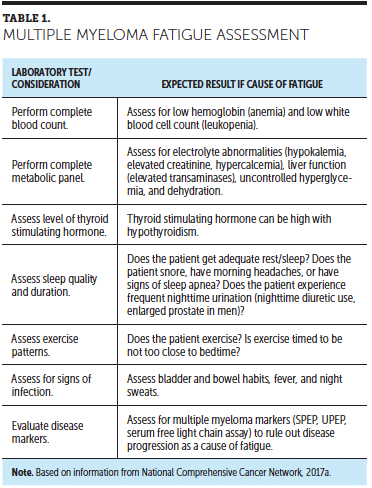
Interventions
The optimal intervention for fatigue is prevention. Because so many factors may contribute to CRF, attention should focus on identifying correctable conditions. Clinical issues, such as nutritional deficiencies, anemia, pain, emotional distress, and sleep disturbances, should be evaluated and addressed to avoid their concomitant effects of CRF (Campos, Hassan, Riechelmann, & Del Giglio, 2011; NCCN, 2017a).
Managing fatigue should improve overall quality of life and physical, psychological, social, and vocational function. However, because the causes of CRF are poorly understood and are influenced by concomitant diseases and their various treatments, few specific and effective treatments exist (Campos et al., 2011). One important goal is to focus on eliminating stressors and increasing patients’ resistance to stressors. Nonpharmacologic interventions include activity enhancement, such as exercise, psychosocial interventions, integrative therapies, nutritional support, sleep therapy, and energy conservation (Campos et al., 2011; Mortimer et al., 2010; Mustian, Sprod, Janelsins, Peppone, & Mohile, 2012). Managing pain, depression, infection, dehydration, insomnia, anemia, nutritional deficiencies, and electrolyte imbalances may improve fatigue and eliminate other symptoms as well (Campos et al., 2011; NCCN, 2017a).
Exercise and mobility enhancement are the most effective nonpharmacologic interventions that improve CRF (Carayol et al., 2013; Coleman et al., 2011; Mishra et al., 2012). A meta-analysis of the effect of exercise on CRF reported an overall benefit during and after cancer treatment (Carayol et al., 2013). The Oncology Nursing Society also recommends exercise to treat CRF (Mitchell, 2011). Encouraging and supporting patient activity and establishing a regular exercise program may prevent and treat fatigue (NCCN, 2017a).
Anxiety and depression can increase symptoms of fatigue (NCCN, 2017a). A meta-analysis reported that behavioral interventions, including behavioral therapy, cognitive therapy, education, relaxation techniques, counseling, or social support reduced fatigue among patients with breast cancer during and after treatment (Duijts, Faber, Oldenburg, van Beurden, & Aaronson, 2011).
Changes in sleep patterns, such as insomnia and hypersomnia, can disrupt sleep (Roscoe et al., 2007) and contribute to CRF. Nonpharmacologic interventions to improve sleep include cognitive behavioral therapy, patient education, and exercise. Cognitive behavioral techniques include sleep restriction, sleep hygiene, and stimulus control. Stimulus control includes going to bed and waking up at the same time each day and getting out of bed after 20 minutes if still awake. Sleep restriction in the form of avoiding long or late afternoon naps, as well as limiting total time spent sleeping, is also considered an important strategy in managing fatigue (Roscoe et al., 2007).
Sleep hygiene involves altering basic lifestyle habits that influence sleep. Examples of lifestyle changes include limiting caffeine, alcohol, and tobacco consumption; relaxing before bedtime; taking warm baths; and getting adequate exercise. Cognitive behavioral interventions include breathing control, progressive muscle relaxation, guided imagery, and complementary therapies, such as massage therapy, yoga, and stress reduction using mindfulness (Berger, Gerber, & Mayer, 2012; NCCN, 2017a). Strategies for conserving energy may also be helpful.
Nutritional consultation can help manage deficiencies caused by anorexia, diarrhea, nausea, and vomiting. Hydration and replacing deficient electrolytes, iron, and folic acid can also prevent or treat fatigue (NCCN, 2017a).
Although the use of psychostimulants, such as methylphenidate and dexmethylphenidate, can improve fatigue, treatments work better when combined with nonpharmacologic interventions, such as exercise and complementary alternative therapies (Campos et al., 2011; NCCN, 2017a). Corticosteroids can be prescribed to patients with palliative intent to treat fatigue during end-of-life care (Yennurajalingam et al., 2013). No medications specific for CRF are approved by the U.S. Food and Drug Administration (FDA). However, certain drugs are approved for treating possible concomitant, contributing diseases that affect fatigue (Minton, Richardson, Sharpe, Hotopf, & Stone, 2011). The antidepressant paroxetine, a selective serotonin reuptake inhibitor, improves depression and mood, but its effects on CRF are mixed. Other antidepressants, such as bupropion, sertraline, and venlafaxine, have also had mixed results in controlling CRF (Mitchell, 2011).
Seven meta-analyses concluded that erythropoiesis-stimulating agents may improve CRF in patients with hemoglobin concentrations of less than 10 g/dl, but the effects are small (Mitchell, 2011). Data also suggest that these agents are associated with marked risks of hypertension and thrombosis, and may reduce disease control and overall survival (Mitchell, 2011). However, treating anemia, including via transfusions in some cases, may reduce fatigue and substantially improve quality of life.
Dexamethasone may improve CRF (Yennurajalingam et al., 2013). A randomized trial of 84 patients tested dexamethasone for treating CRF. At day 15, the mean score of the 13-item Functional Assessment of Chronic Illness Therapy–Fatigue instrument was significantly better in the dexamethasone group than in the placebo group (9 versus 3.1 on a scale of 1–13, where 13 is no fatigue). The improvement in total quality of life was also significantly higher in the dexamethasone group. However, dexamethasone is associated with side effects, such as hyperglycemia, avascular necrosis, muscle weakness, emotional lability, inattention/hyperactivity, mania, psychosis, and altered sleep patterns (King & Faiman, 2017; Yennurajalingam et al., 2013).
Evaluating fatigue in patients with MM, particularly those with known treatable factors as described previously, is essential at each clinic visit and after each intervention (NCCN, 2017a). Fatigue occurs in the context of multiple symptoms, and these symptoms may act synergistically to increase symptomatology (NCCN, 2017a). Fatigue may improve as the disease becomes better controlled, a relationship that can help patients better understand the symptoms and management of CRF.
Evidence-Based Recommendations for Fatigue
Level of Evidence I
• Routinely evaluate patients for fatigue (NCCN, 2017a).
• If fatigue is present, rule out and correct other organic causes of fatigue, such as anemia, thyroid dysfunction, sleep apnea, or vitamin deficiencies.
• Exercise is effective in the nonpharmacologic management of fatigue.
• Effective interventions include establishing adequate nutrition, reducing stress, and adopting sleep hygiene techniques.
• Pharmacostimulants, such as methylphenidate and dexmethylphenidate, can improve severe fatigue and work better when combined with nonpharmacologic interventions, such as exercise and complementary alternative therapies (Campos et al., 2011; NCCN, 2017a).
• Corticosteroids can be used with palliative intent among patients with severe fatigue and during end-of-life care (Yennurajalingam et al., 2013).
Sexual Dysfunction
Sexual dysfunction in patients with cancer may be caused by the disease, chemotherapeutic agents, surgical procedures, hormonal therapy, medications, and comorbid conditions and can interfere with intimate relationships (Richards, Bertolotti, Doss, & McCullagh, 2011). Providers should routinely ask patients with MM about sexual function and dysfunction because patients usually do not volunteer information about these topics despite citing sexuality as a major concern (Goncalves & Groninger, 2015; Osborne et al., 2014) (see Figures 6 and 7). Sexual dysfunction is an issue only if the patient regards it as one. Sexual dysfunction is not a part of normal aging. Therefore, once a problem with sexual function has been identified, possible causes should be investigated.
Sexual Dysfunction in Men
Sexual dysfunction in men includes erectile dysfunction, changes in libido, premature ejaculation, and delayed or inhibited ejaculation (Cunningham & Khera, 2014). Erectile dysfunction has been reported in about 50% of otherwise healthy men aged 40–70 years (Patel, Halls, & Patel, 2012). In 10%–20% of men with erectile dysfunction, the cause is thought to be solely psychological. How many men with MM experience erectile dysfunction is unknown (Sadovsky et al., 2010).
Some agents used to treat MM may lead to sexual dysfunction (Celgene, 2017b; Sadovsky et al., 2010). Alkylating agents, such as melphalan (particularly in high doses) and cyclophosphamide, may decrease semen production, contribute to erectile dysfunction, and reduce desire (Sadovsky et al., 2010; Thygesen, Schjødt, & Jarden, 2012). Peripheral neuropathy is one of the more common side effects of thalidomide and bortezomib, and both drugs may damage small nerve fibers, causing erectile dysfunction (Delforge et al., 2010). In addition, lenalidomide has been associated with erectile dysfunction in clinical trials (Celgene, 2017a). In addition to erectile dysfunction, bortezomib may cause testicular swelling, pain, and peripheral neuropathy (Richards et al., 2011). Steroid therapy used to treat MM may reduce desire and cause erectile dysfunction (Kalantaridou, Calis, et al., 2006; Kalantaridou, Naka, et al., 2006). Steroid therapy may also cause hyperglycemia, which can also reduce sexual function (Faiman, Bilotti, Mangan, & Rogers, 2008; Richards et al., 2011). In men without prostate cancer, comorbid conditions, such as diabetes, hypertension, thyroid disorders, renal disease, cardiovascular disease, pain, and disturbances in body image, may reduce sexual function (Richards et al., 2011).
[[{"type":"media","view_mode":"media_original","fid":"36041","field_deltas":{"3":{}},"link_text":null,"fields":{},"attributes":{"height":"646","width":"749","class":"media-image media-element file-media-original","data-delta":"3"}}]]
Sexual Dysfunction in Women
About half of women with MM report sexual dysfunction lasting more than a month, but only about 20% seek treatment for it (Srivastava, Thakar, & Sultan, 2008). Women may experience hypoactive arousal, painful intercourse, loss of desire, and difficulties having an orgasm. The most common report of sexual dysfunction is loss of desire (Fourcroy, 2003; Srivastava et al., 2008).
In women, sexual dysfunction may be caused by medications, hormonal therapy, ovarian failure, pain, body image changes, and changes in interpersonal relationships. Treatments, such as stem cell transplantation, alkylating agents, steroids, and the use of birth control, may reduce sexual function. The effect of new agents, such as pomalidomide, carfilzomib, ixazomib, daratumumab, elotuzumab, and panobinostat, on sexual function is unknown. In stem cell transplantation, women tend to not recover baseline sexual function, whereas men return to baseline function within two to three years after transplantation (Li et al., 2015). Alkylating agents may cause ovarian failure, leading to vaginal dryness, decreased desire, and painful intercourse (Sadovsky et al., 2010). Several factors increase the risk for sexual dysfunction in men and women after stem cell transplantation, including graft-versus-host disease, cardiac complications, and prescription medications (Li et al., 2015).
Assessment
Properly assessing sexual function is difficult, given the reluctance of patients and caregivers to discuss the topic and a lack of provider training and standardized questionnaires on sexual function. Two questionnaires have been validated in patients with cancer that could be relevant to patients with MM, the International Index of Erectile Function and the Female Sexual Function Index (Bober & Varela, 2012). In addition, reviewing medications and comorbid conditions to determine whether they may be contributing to the dysfunction is essential (McVary, 2007; Richards et al., 2011; Srivastava et al., 2008).
Treating Male Sexual Dysfunction
Erectile dysfunction may be reversed with phosphodiesterase type-5 inhibitors. However, these medications are contraindicated in men receiving nitrate therapy because they may cause hypotension (McVary, 2007). Nonpharmacologic interventions for erectile dysfunction include testosterone replacement, vacuum devices, surgery, and psychotherapy (Bruner & Calvano, 2007; McVary, 2007; Richards et al., 2011). The use of testosterone therapy in men with hypogonadism is controversial and is contraindicated in men with a history of prostate cancer (Hackett, 2016). The risks and benefits should be discussed with the patient before starting therapy (Hackett, 2016). Although intracavernous and transurethral injections are used to treat erectile dysfunction, their use in men with MM is contraindicated by the risk of priapism (i.e., prolonged erection) (McVary, 2007; Richards et al., 2011). Healthcare providers may refer patients to specialists in erectile dysfunction (see Figure 8). 
Treating Female Sexual Dysfunction
Options to treat female sexual dysfunction are limited. Medications as treatment include flibanserin. Approved by the FDA in 2015, flibanserin is a treatment for premenopausal women reporting a low desire for sex (Valent Pharmaceuticals, 2016). Potential side effects include low blood pressure or fainting, nausea, dizziness, and headache. Women taking flibanserin must avoid alcohol. Bremelanotide is a cyclic 7 amino acid melanocortin receptor agonist with high affinity for the type 4 receptor and the potential to modulate brain pathways involved in sexual response. Women taking bremelanotide in a randomized placebo-controlled study had a greater number of satisfying sexual events and improved sexual desire and arousal than that of women receiving placebo (Clayton et al., 2016). This drug is not yet approved by the FDA (Safarinejad, 2008).
Vaginal lubricants are recommended for the initial treatment of vaginal dryness (Goncalves & Groninger, 2015). Women should be encouraged to discuss vaginal dryness with their gynecologist to determine the best treatment for their sexual dysfunction (Goncalves & Groninger, 2015).
Evidence-Based Recommendations for Sexual Dysfunction
Level of Evidence I
• Routinely assess patients for sexual dysfunction.
• For men, evidence supports the use of nonpharmacologic interventions, such as vacuum devices, surgery, and psychotherapy (Bruner & Calvano, 2007; McVary, 2007; Richards et al., 2011).
• The use of testosterone therapy in men with hypogonadism is controversial and contraindicated in men with a history of prostate cancer (Hackett, 2016). The risks and benefits should be discussed.
• The evidence also supports the use of phosphodiesterase type-5 inhibitors in men experiencing erectile dysfunction (McVary, 2007).
• Flibanserin is approved for use in premenopausal women who experience a lack of desire, and the drug can be recommended (Valent Pharmaceuticals, 2016). 
Conclusion
Patients with MM will often receive several treatment regimens throughout their illness. The aims of anti-MM therapy are to control the disease, prolong survival, and increase quality of life. However, appropriate management of patients with MM requires ongoing assessment of several concurrent symptoms caused by the disease, its treatment, or both. Distress, fatigue, and sexual dysfunction have been reported to adversely affect psychosocial quality of life. If untreated, they may lead to unnecessary suffering, family burden, frequent visits to the healthcare provider, added stress on the healthcare team, and difficulties in treatment decision making and adherence. Nursing and clinical care can benefit patients and caregivers through critical assessment and interventions.
The authors gratefully acknowledge Rafat Abonour, MD, Brian G.M. Durie, MD, and Diane P. Moran, RN, MA, EdM, at the International Myeloma Foundation for their review of this manuscript.
About the Author(s)
Donna Catamero, ANP-BC, OCN®, CCRC, is a nurse practitioner at Mount Sinai Hospital in New York, NY; Kimberly Noonan, RN, MS, CNP, AOCN®, is a nurse practitioner at Dana-Farber Cancer Institute in Boston, MA; Tiffany Richards, PhD, ANP-BC, is a nurse practitioner at the University of Texas MD Anderson Cancer Center in Houston; Beth Faiman, PhD, MSN, APRN-BC, AOCN®, is a nurse practitioner in the Department of Hematology and Medical Oncology at the Cleveland Clinic Taussig Cancer Institute in Ohio; Cindy Manchulenko, RN, BN, MSN, is a clinical trials project manager at the Vancouver Coastal Health Authority in British Columbia, Canada; Hollie Devine, MSN, RN, ANP-BC, is a nurse practitioner at the Ohio State University Comprehensive Cancer Center–Arthur G. James Cancer Hospital and Richard J. Solove Research Institute in Columbus; Page Bertolotti, RN, BSN, OCN®, is a clinical nurse III at the Samuel Oschin Cancer Center at Cedars-Sinai Medical Center in Los Angeles, CA; and Charise Gleason, MSN, ANP-C, AOCNP®, is a nurse practitioner chief at the Emory Winship Cancer Institute in Atlanta, GA. The authors take full responsibility for this content. This supplement was supported by the International Myeloma Foundation, with funding from Celgene Corporation, Karyopharm Therapeutics, and Takeda Oncology. Writing and editorial support was provided by Eubio Medical Communications. Catamero has previously consulted for Celgene Corporation and has previously served on speakers bureaus for Amgen, Celgene Corporation, Janssen Pharmaceuticals, Biotech, and Takeda Oncology. Richards has previously consulted for Celgene Corporation and Takeda Oncology. Faiman consults and serves on speakers bureaus for Amgen, Bristol-Myers Squibb, Celgene Corporation, and Takeda Oncology, and has received support from Celgene Corporation and Takeda Oncology. Manchulenko has previously consulted for Amgen, Celgene Corporation, Janssen Pharmaceuticals, and Takeda Oncology, and serves on speakers bureaus for Celgene Corporation and Janssen Pharmaceuticals. Bertolotti serves on speakers bureaus for Celgene Corporation and Takeda Oncology. The article has been reviewed by independent peer reviewers to ensure that it is objective and free from bias. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society. Catamero can be reached at donna.catamero@mountsinai.org, with copy to CJONEditor@ons.org. (Submitted June 2017. Accepted August 1, 2017.)
References
Adler, N.E., & Page, A.E. (Eds.). (2008). Cancer care for the whole patient: Meeting psychosocial health needs. Washington, D.C.: National Academies Press.
American College of Surgeons. (2012). Cancer program standards 2012: Ensuring patient-centered care. Retrieved from http://bit.ly/1B9K7sp
Amgen. (2017). Kyprolis® (carfilzomib) [Package insert]. Retrieved from http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/kyprolis…
Berger, A.M., Gerber, L.H., & Mayer, D.K. (2012). Cancer-related fatigue: Implications for breast cancer survivors. Cancer, 118(Suppl. 8), 2261–2269. https://doi.org/10.1002/cncr.27475
Bober, S.L., & Varela, V.S. (2012). Sexuality in adult cancer survivors: Challenges and intervention. Journal of Clinical Oncology, 30, 3712–3719.
Boland, E.G., Boland, J.W., Ezaydi, Y., Greenfield, D.M., Ahmedzai, S.H., & Snowden, J.A. (2014). Holistic needs assessment in advanced, intensively treated multiple myeloma patients. Supportive Care in Cancer, 22, 2615–2620. https://doi.org/10.1007/s00520-014-2231-2
Bruner, D.W., & Calvano, T. (2007). The sexual impact of cancer and cancer treatments in men. Nursing Clinics of North America, 42, 555–580. https://doi.org/10.1016/j.cnur.2007.07.005
Campos, M.P., Hassan, B.J., Riechelmann, R., & Del Giglio, A. (2011). Cancer-related fatigue: A practical review. Annals of Oncology, 22, 1273–1279. https://doi.org/10.1093/annonc/mdq458
Carayol, M., Bernard, P., Boiché, J., Riou, F., Mercier, B., Cousson-Gélie, F., . . . Ninot, G. (2013). Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: What is the optimal dose needed? Annals of Oncology, 24, 291–300.
Celgene. (2016). Pomalyst® (pomalidomide) [Package insert]. Retrieved from http://www.celgene.com/content/uploads/pomalyst-pi.pdf
Celgene. (2017a). Revlimid® (lenalidomide) [Package insert]. Retrieved from http://www.celgene.com/content/uploads/revlimid-pi.pdf
Celgene. (2017b). Thalomid® (thalidomide) [Package insert]. Retrieved from http://www.celgene.com/content/uploads/thalomid-pi.pdf
Clayton, A.H., Althof, S.E., Kingsberg, S., DeRogatis, L.R., Kroll, R., Goldstein, I., . . . Portman, D.J. (2016). Bremelanotide for female sexual dysfunctions in premenopausal women: A randomized, placebo-controlled dose-finding trial. Women’s Health, 12, 325–337.
Clayton, A., & Ramamurthy, S. (2008). The impact of physical illness on sexual dysfunction. Advances in Psychosomatic Medicine, 29, 70–88. https://doi.org/10.1159/000126625
Coleman, E.A., Goodwin, S.A., Coon, S.K., Richards, K., Enderlin, C., Kennedy, R., . . . Barlogie, B. (2011). Fatigue, sleep, pain, mood, and performance status. Cancer Nursing, 34, 219–227.
Cormican, O., & Dowling, M. (2016). Managing relapsed myeloma: The views of patients, nurses and doctors. European Journal of Oncology Nursing, 23, 51–58.
Cunningham, G.R., & Khera, M. (2015). Evaluation of male sexual dysfunction. UptoDate. Retrieved from http://www.uptodate.com/contents/evaluation-of-male-sexual-dysfunction
Dahan, J.F., & Auerbach, C.F. (2006). A qualitative study of the trauma and posttraumatic growth of multiple myeloma patients treated with peripheral blood stem cell transplant. Palliative and Supportive Care, 4, 365–387.
Delforge, M., Bladé, J., Dimopoulos, M.A., Facon, T., Kropff, M., Ludwig, H., . . . Sonneveld P. (2010). Treatment-related peripheral neuropathy in multiple myeloma: The challenge continues. Lancet Oncology, 11, 1086–1095. https://doi.org/10.1016/S1470-2045(10)70068-1
Derogatis, L.R., Morrow, G.R., Fetting, J., Penman, D., Piasetsky, S., Schmale, A.M., . . . Carnicke, C.L. (1983). The prevalence of psychiatric disorders among cancer patients. JAMA, 249, 751–757.
Duijts, S.F., Faber, M.M., Oldenburg, H.S., van Beurden, M., & Aaronson, N.K. (2011). Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—A meta-analysis. Psycho-Oncology, 20, 115–126. https://doi.org/10.1002/pon.1728
Faiman, B., Bilotti, E., Mangan, P.A., & Rogers, K. (2008). Steroid-associated side effects in patients with multiple myeloma: Consensus statement of the IMF Nurse Leadership Board. Clinical Journal of Oncology Nursing, 12(Suppl. 3), S53–S63.
Felbel, S., Meerpohl, J.J., Monsef, I., Engert, A., & Skoetz, N. (2014). Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database of Systematic Reviews, 6, CD010146. https://doi.org/10.1002/14651858.CD010146.pub2
Fourcroy, J.L. (2003). Female sexual dysfunction. Drugs, 63, 1445–1457.
Goncalves P., & Groninger, H. (2015). Sexual dysfunction in cancer patients and survivors #293. Journal of Palliative Medicine, 18, 714–715. https://doi.org/10.1089/jpm.2015.0207
Hackett, G. (2016). An update on the role of testosterone replacement therapy in the management of hypogonadism. Therapeutic Advances in Urology, 8, 147–160.
Harrison, J.D., Young, J.M., Price, M.A., Butow, P.N., & Solomon, M.J. (2009). What are the unmet supportive care needs of people with cancer? A systematic review. Supportive Care in Cancer, 17, 1117–1128. https://doi.org/10.1007/s00520-009-0615-5
Holland, J.C., & Alici, Y. (2010). Management of distress in cancer patients. Journal of Supportive Oncology, 8, 4–12.
Holland, J.C., & Bultz, B.D. (2007). The NCCN guideline for distress management: A case for making distress the sixth vital sign. Journal of the National Comprehensive Cancer Network, 5(1), 3–7.
Holland, J.C., Greenberg, D.B., & Hughes M.K. (Eds.). (2006). Quick reference for oncology clinicians: The psychiatric and psychological dimensions of cancer symptom management. Charlottesville, VA: International Psyco-Oncology Society (IPOS) Press.
Horneber, M., Fischer, I., Dimeo, F., Rüffer, J.U., & Weis, J. (2012). Cancer-related fatigue. Deutsches Ärzteblatt International, 109(9), 161–172.
Horwitz, A.V. (2007). Transforming normality into pathology: The DSM and the outcomes of stressful social arrangements. Journal of Health and Social Behavior, 48, 211–222.
Husson, O., Thong, M.S., Mols, F., Oerlemans, S., Kaptein, A.A., & van de Poll-Franse, L.V. (2013). Illness perceptions in cancer survivors: What is the role of information provision? Psycho-Oncology, 22, 490–498. https://doi.org/10.1002/pon.3042
Howell, D., Keller-Olaman, S., Oliver, T.K., Hack, T.F., Broadfield, L., Biggs, K., . . . Olson, K. (2013). A pan-Canadian practice guideline and algorithm: Screening, assessment, and supportive care of adults with cancer-related fatigue. Current Oncology, 23(3), e233–e246.
Jacobsen, P.B., Donovan, K.A., Swaine, Z.N., & Watson, I.S. (2006). Management of anxiety and depression in adult cancer patients: Toward an evidence-based approach. In A.E. Chang, P.A. Ganz, D.F. Hayes, T. Kinsella, H.I. Pass, J.H. Schiller, R. Stone, & V. Strecher (Eds.), Oncology (pp. 1561–1588). New York, NY: Springer-Verlag.
Kalantaridou, S.N., Calis, K.A., Vanderhoof, V.H., Bakalov, V.K., Corrigan, E.C., Troendle, J.F., & Nelson, L.M. (2006). Testosterone deficiency in young women with 46,XX spontaneous premature ovarian failure. Fertility and Sterility, 86, 1475–1482.
Kalantaridou, S.N., Naka, K.K., Bechlioulis, A., Makrigiannakis, A., Michalis, L., & Chrousos, G.P. (2006). Premature ovarian failure, endothelial dysfunction and estrogen–progestogen replacement. Trends in Endocrinology and Metabolism, 17, 101–109.
Kelly, M., & Dowling, M. (2011). Patients’ lived experience of myeloma. Nursing Standard, 25(28), 38–44. https://doi.org/10.7748/ns2011.03.25.28.38.c8397
King, T., & Faiman, B. (2017). Steroid-associated side effects: A symptom management update on multiple myeloma treatment. Clinical Journal of Oncology Nursing, 21(2), 240–249.
Lamers, J., Hartmann, M., Goldschmidt, H., Brechtel, A., Hillengass, J., & Herzog, W. (2013). Psychosocial support in patients with multiple myeloma at time of diagnosis: Who wants what? Psycho-Oncology, 22, 2313–2320. https://doi.org/10.1002/pon.3284
Li, Z., Mewawalla, P., Stratton, P., Yong, A.S., Shaw, B.E., Hashmi, S., . . . Rovó, A. (2015). Sexual health in hematopoietic stem cell transplant recipients. Cancer, 121, 4124–4131.
Maher, K., & de Vries, K. (2011). An exploration of the lived experiences of individuals with relapsed multiple myeloma. European Journal of Cancer Care, 20, 267–275.
McVary, K.T. (2007). Clinical practice. Erectile dysfunction. New England Journal of Medicine, 357, 2472–2481. https://doi.org/10.1056/NEJMcp067261
Miceli, T., Lilleby, K., Noonan, K., Kurtin, S., Faiman, B., & Mangan, P.A. (2013). Autologous hematopoietic stem cell transplantation for patients with multiple myeloma: An overview for nurses in community practice. Clinical Journal of Oncology Nursing, 17(Suppl.), 13–24.
Millennium Pharmaceuticals. (2017). Velcade® (bortezomib) [Package insert]. Retrieved from http://www.velcade.com/files/PDFs/VELCADE_PRESCRIBING_INFORMATION.pdf
Minton, O., Richardson, A., Sharpe, M., Hotopf, M., & Stone, P.C. (2011). Psychostimulants for the management of cancer-related fatigue: A systematic review and meta-analysis. Journal of Pain and Symptom Management, 41, 761–767.
Mishra, S.I., Scherer, R.W., Snyder, C., Geigle, P.M., Berlanstein, D.R., & Topaloglu, O. (2012). Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database of Systematic Reviews, 8, CD008465.
Mitchell, S. (2011). Cancer-related fatigue. In C.H. Yarbro, D. Wujcik, & B.H. Gobel (Eds.), Cancer nursing: Principles and practice (7th ed., pp. 772–791). Burlington, MA: Jones & Bartlett.
Mortimer, J.E., Barsevick, A.M., Bennett, C.L., Berger, A.M., Cleeland, C., DeVader, S.R., . . . Rugo, H.S. (2010). Studying cancer-related fatigue: Report of the NCCN scientific research committee. Journal of the National Comprehensive Cancer Network, 8, 1331–1339.
Mustian, K.M., Sprod, L.K., Janelsins, M., Peppone, L.J., & Mohile, S. (2012). Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: A review. Oncology and Hematology Review, 8, 81–88.
National Comprehensive Cancer Network. (2017a). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Cancer-related fatigue [v.2.2017]. Retrieved from http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf
National Comprehensive Cancer Network. (2017b). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Distress management [v.1.2017]. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf
Olivieri, A., Capelli, D., Montanari, M., Brunori, M., Massidda, D., Poloni, A., . . . Leoni, P. (2001). Very low toxicity and good quality of life in 48 elderly patients autotransplanted for hematological malignancies: A single center experience. Bone Marrow Transplantation, 27, 1189–1195. https://doi.org/10.1038/sj.bmt.1703034
Oncology Nursing Society. (2017). Putting Evidence Into Practice: Fatigue. Retrieved from https://www.ons.org/practice-resources/pep/fatigue
Osborne, T.R., Ramsenthaler, C., de Wolf-Linder, S., Schey, S.A., Siegert, R.J., Edmonds, P.M., & Higginson, I.J. (2014). Understanding what matters most to people with multiple myeloma: A qualitative study of views on quality of life. BMC Cancer, 14, 496.
Patel, D.V., Halls, J., & Patel, U. (2012). Investigation of erectile dysfunction. British Journal of Radiology, 85(Spec No. 1), S69–S78. https://doi.org/10.1259/bjr/20361140
Phillips, M.R. (2009). Is distress a symptom of mental disorders, a marker of impairment, both or neither? World Psychiatry, 8, 91–92.
Pirl, W.F. (2004). Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. Monographs. National Cancer Institute, 32, 32–39.
Potrata, B., Cavet, J., Blair, S., Howe, T., & Molassiotis, A. (2010). ‘Like a sieve’: An exploratory study on cognitive impairments in patients with multiple myeloma. European Journal of Cancer Care, 19, 721–728. https://doi.org/10.1111/j.1365-2354.2009.01145.x
Potrata, B., Cavet, J., Blair, S., Howe, T., & Molassiotis, A. (2011). Understanding distress and distressing experiences in patients living with multiple myeloma: An exploratory study. Psycho-Oncology, 20, 127–134. https://doi.org/10.1002/pon.1715
Richards, T.A., Bertolotti, P.A., Doss, D., & McCullagh, E.J. (2011). Sexual dysfunction in multiple myeloma: Survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clinical Journal of Oncology Nursing, 15(Suppl.), 53–65.
Roscoe, J.A., Kaufman, M.E., Matteson-Rusby, S.E., Palesh, O.G., Ryan, J.L., Kohli, S., . . . Morrow, G. R. (2007). Cancer-related fatigue and sleep disorders. Oncologist, 12(Suppl. 1), 35–42.
Sadovsky, R., Basson, R., Krychman, M., Morales, A.M., Schover, L., Wang, R., & Incrocci, L. (2010). Cancer and sexual problems. Journal of Sexual Medicine, 7, 349–373.
Safarinejad, M.R. (2008). Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: A double-blind placebo-controlled, fixed dose, randomized study. Journal of Sexual Medicine, 5, 887–897.
Schneiderman, N., Ironson, G., & Siegel, S. D. (2005). Stress and health: Psychological, behavioral, and biological determinants. Annual Review of Clinical Psychology, 1, 607–628. https://doi.org/10.1146/annurev.clinpsy.1.102803.144141
Sherman, A.C., Simonton, S., Latif, U., Spohn, R., & Tricot, G. (2004). Psychosocial adjustment and quality of life among multiple myeloma patients undergoing evaluation for autologous stem cell transplantation. Bone Marrow Transplantation, 33, 955–962.
Smith, L., McCourt, O., Henrich, M., Paton, P., Yong, K., Wardle, J., & Fisher, A. (2015). Multiple myeloma and physical activity: A scoping review. BMJ Open, 5(11), e009576.
Srivastava, R., Thakar, R., & Sultan, A. (2008). Female sexual dysfunction in obstetrics and gynecology. Obstetrical and Gynecological Survey, 63, 527–537.
Stephens, M., McKenzie, H., & Jordens, C.F. (2014). The work of living with a rare cancer: Multiple myeloma. Journal of Advanced Nursing, 70, 2800–2809.
Stone, P.C., & Minton, O. (2008). Cancer-related fatigue. European Journal of Cancer, 44, 1097–1104. https://doi.org/10.1016/j.ejca.2008.02.037
Strasser-Weippl, K., & Ludwig, H. (2008). Psychosocial QOL is an independent predictor of overall survival in newly diagnosed patients with multiple myeloma. European Journal of Hematology, 81, 374–379. https://doi.org/10.1111/j.1600-0609.2008.01126.x
Swash, B., Hulbert-Williams, N., & Bramwell, R. (2014). Unmet psychosocial needs in haematological cancer: A systematic review. Supportive Care in Cancer, 22, 1131–1141.
Thygesen, K.H., Schjødt, I., & Jarden, M. (2012). The impact of hematopoietic stem cell transplantation on sexuality: A systematic review of the literature. Bone Marrow Transplantation, 47, 716–724. https://doi.org/10.1038/bmt.2011.169
Trask, P.C., Paterson, A., Riba, M., Brines, B., Griffith, K., Parker, P., . . . Ferrara, J. (2002). Assessment of psychological distress in prospective bone marrow transplant patients. Bone Marrow Transplantation, 29, 917–925. https://doi.org/10.1038/sj.bmt.1703557
Tomlinson, J. (1998). ABC of sexual health: Taking a sexual history. BMJ, 317, 1573–1576.
Valent Pharmaceuticals. (2016). Addyi® (flibanserin) [Package insert]. Retrieved from https://www.addyirems.com/AddyiUI/rems/pdf/prescribingInformation.pdf
Vlossak, D., & Fitch, M.I. (2008). Multiple myeloma: the patient’s perspective. Canadian Oncology Nursing Journal, 18(3), 141–151.
Weisman, A.D., & Worden, J.W. (1977). The existential plight in cancer: Significance of the first 100 days. International Journal of Psychiatry in Medicine, 7, 1–15.
Williams, S., & Dale, J. (2006). The effectiveness of treatment for depression/depressive symptoms in adults with cancer: A systematic review. British Journal of Cancer, 94, 372–390. https://doi.org/10.1038/sj.bjc.6602949
Yennurajalingam, S., Frisbee-Hume, S., Palmer, J.L., Delgado-Guay, M.O., Bull, J., Phan, A.T., . . . Bruera, E. (2013). Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. Journal of Clinical Oncology, 31, 3076–3082. https://doi.org/10.1200/JCO.2012.44.4661
Zabora, J., Buzaglo, J., Kennedy, V., Richards, T., Schapmire, T., Zebrack, B., & Ghobrial, I.M. (2015). Clinical perspective: Linking psychosocial care to the disease continuum in patients with multiple myeloma. Palliative and Supportive Care, 13, 829–838. https://doi.org/10.1017/S1478951514000649




